Staudinger ketene cycloaddition
The Staudinger ketene cycloaddition (also known as Staudinger synthesis ) is a name reaction in organic chemistry , which the German chemist Hermann Staudinger first reported in 1907. This reaction can produce β-lactams . It should not be confused with the Staudinger reaction , which is about the reduction of azides to amines .
Overview reaction
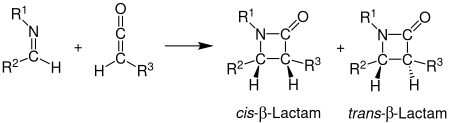
In the Staudinger synthesis, an imine and a ketene react to form a β-lactam under [2 + 2] cycloaddition :
General
The Staudinger ketene cycloaddition is a synthesis that has received a lot of attention to this day. Papers are still being written on the stereochemistry of this reaction. Diethyl ether was originally used as the solvent for the imine and petroleum ether for the ketene . The product precipitates out on heating and can be filtered off and washed after a few hours.
Reaction mechanism
The reaction mechanism of the Staudinger synthesis could look like this. In the first step, the lone pair of electrons on the nitrogen of imine 1 attacks nucleophilically on the central carbon atom of the ketene group of compound 2 . The zwitterion 3 is created . An intramolecular nucleophilic attack on the carbon of the imine group leads to ring closure. The β-lactam 4 is formed :
Stereochemistry
Understanding the stereoselectivity in the formation of β-lactams using the Staudinger synthesis is still a problem that has not been fully resolved. However, it is assumed that the direction from which the imine attacks and the isomerization determine the stereoselectivity. It was also observed that ketenes with strong electron donors as substituents mostly ensure the formation of a cis product, and ketenes with strong electron acceptors as substituents favor the production of the trans product. Many theories on the stereochemistry of the Staudinger synthesis have been developed, but none have been ascribed general validity. In the [2 + 2] cycloaddition of an imine and a ketene, the cis and trans products can be formed.
Variations
In the thio-Staudinger cycloaddition , a thioketene reacts instead of the oxoketene. The reaction of a ketene with an alkene produces a cyclobutanone , a carbonyl compound produces a β-lactone and a carbodiimide can produce a 4-imino-β-lactam. It must be noted that unstable Staudinger products can react further to form secondary products.
One-pot reaction
A one-pot reaction according to the Staudinger synthesis was described in 2014 by Doyle's team. In this synthesis, an azide and two diazo compounds react with rhodium (II) acetate as the catalyst and with dichloromethane as the solvent to form a β-lactam. The reaction takes place over 3 hours at room temperature and gives a yield of approx. 99%.
Sulfa-Staudinger cycloaddition
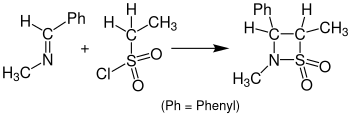
The reaction with sulfenes instead of ketenes, which leads to the formation of β-sultams , is called the sulfa-Staudinger cycloaddition . Solvent and reaction temperature can have a significant influence on the yields. The implementation in dichloromethane with the addition of triethylamine at 20 ° C. has proven successful . The temperature should not be set too low during the actual addition. Occasionally, tetrahydrofuran is used as a solvent. The following figure shows the reaction of benzylidene (methyl) amine with ethanesulfonic acid chloride to give the corresponding β-sultam.
application
Almost 40 years after the publication of the Staudinger ketene cycloaddition, it was only given wider attention. At that time, penicillin was found to contain β-lactams. In addition, Staudinger's report began research into the [2 + 2] cycloaddition 20 years before the Diels-Alder reaction was published. The Staudinger synthesis is one of the most fundamental and one of the most versatile methods for the production of β-lactams. These play a very important role in pharmaceutical chemistry as well as in synthetic chemistry.
Individual evidence
- ^ A b Hermann Staudinger: On the knowledge of the ketenes. Diphenylketene . In: Justus Liebig's Annals of Chemistry . tape 356 , no. 1-2 . John Wiley & Sons, Inc., 1907, p. 51-123 , doi : 10.1002 / jlac.19073560106 .
- ↑ a b c Lei Jiao, Yong Liang, Jiaxi Xu: Origin of the Relative Stereoselectivity of the β-Lactam Formation in the Staudinger Reaction . In: Journal of the American Chemical Society . tape 128 , no. 18 , 2006, p. 6060–6069 , doi : 10.1021 / ja056711k .
- ↑ Wei He et al .: Sterically controlled diastereoselectivity in thio-Staudinger cycloadditions of alkyl / alkenyl / aryl-substituted thioketenes . In: Organic & Biomolecular Chemistry . 2017, p. 5541-5548 , doi : 10.1039 / C7OB01214D .
- ^ Jie Jack Li: Name reactions. A collection of detailed reaction mechanisms . 3. Edition. Springer-Verlag, Berlin 2006, ISBN 978-3-540-30030-4 , pp. 561-562 , doi : 10.1007 / 3-540-30031-7 .
- ↑ Michael D. Mandler, Phong M. Truong, Peter Y. Zavalij, Michael P. Doyle: Catalytic Conversion of Diazocarbonyl Compounds to Imines: Applications to the Synthesis of Tetrahydropyrimidines and β-Lactams . In: Organic Letters . tape 16 , no. 3 , 2014, p. 740-743 , doi : 10.1021 / ol403427s .
- ↑ a b Zhanhui Yang, Ning Chen, Xu Jiaxi: substituent-Controlled Annuloselectivity and Stereoselectivity in the sulfa Staudinger cycloaddition . In: The Journal of Organic Chemistry . tape 80 , no. 7 , 2015, p. 3611-3620 , doi : 10.1021 / acs.joc.5b00312 .
- ↑ Wu Q, Yang Z, Xu J: Temperature-dependent annuloselectivity and stereochemistry in the reactions of methanesulfonyl sulfene with imines . In: Organic & Biomolecular Chemistry . No. 30 , 2016, p. 7258-67 , doi : 10.1039 / c6ob01259k .
- ↑ Thomas T. Tidwell: Hugo (Ugo) Schiff, Schiff Bases, and a Century of β ‐ Lactam Synthesis . In: Angewandte Chemie International Edition . tape 47 , no. 6 . John Wiley & Sons, Inc., 2008, p. 1016-1020 , doi : 10.1021 / ja056711k .