Cyclooctene
| Structural formula | |||||||||
|---|---|---|---|---|---|---|---|---|---|

|
|||||||||
| ( Z ) -cyclooctene (top) and the two enantiomeric ( E ) -cyclooctene (bottom): ( P ) -cyclooctene and ( M ) -cyclooctene | |||||||||
| General | |||||||||
| Surname | Cyclooctene | ||||||||
| other names |
COE |
||||||||
| Molecular formula | C 8 H 14 | ||||||||
| Brief description |
inflammable, colorless, characteristic smelling liquids |
||||||||
| External identifiers / databases | |||||||||
|
|||||||||
| properties | |||||||||
| Molar mass | 110.20 g mol −1 | ||||||||
| Physical state |
liquid |
||||||||
| density |
0.85 g cm −3 |
||||||||
| Melting point |
−16 ° C |
||||||||
| boiling point |
148 ° C |
||||||||
| Vapor pressure |
8 h Pa (20 ° C) |
||||||||
| Refractive index |
1.469 (20 ° C) |
||||||||
| safety instructions | |||||||||
|
|||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||
Cyclooctene is a chemical compound from the group of unsaturated , cyclic hydrocarbons (more precisely the cycloalkenes ).
Extraction and presentation
( Z ) -Cyclooctene is obtained in good yield by hydrogenation of cyclooctatetraene or by Cope elimination of N , N- dimethylcyclooctylamine oxide. The racemate of ( E ) - (+) - cyclooctene and ( E ) - (-) - cyclooctene is formed (in addition to ( Z ) -cyclooctene) by pyrolysis of N , N , N -trimethylcyclooctylammonium hydroxide.
A very elegant route to the synthesis of ( E ) -cyclooctene is a Corey-Winter elimination starting from ( E ) -1,2-cyclooctanediol :
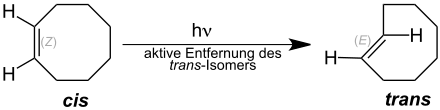
( E ) -cyclooctene can also be obtained from ( Z ) -cyclooctene by a photochemical isomerization . Although the equilibrium here is on the side of the ( Z ) -cyclooctene, the reaction can be driven to completion by removing the ( E ) isomer by complexation with silver.
Technically, it is produced by selective hydrogenation of 1,5-cyclooctadiene , which can be obtained by a process by Wilke by dimerizing 1,3-butadiene .
properties
There are three isomers of cyclooctene : ( Z ) -cyclooctene, ( pR ) - ( E ) -cyclooctene, ( pS ) - ( E ) -cyclooctene. The ring size in cyclooctene just allows the double bond to be either ( E ) - or ( Z ) -configured. ( E ) -cyclooctene is optically active , i.e. chiral , due to the lack of a rotating mirror axis . The p in front of the R / S descriptors indicates that this is a planar chirality. The isomerization reaction can be carried out in an enantiodifferentiating manner using a photosensitizer , that is, one enantiomer of the E isomer of cyclooctene is obtained in excess.
use
The ring-opening polymerization of ( Z ) -cyclooctene gives polyoctenamer , which is used as a component in elastomers . The ozonolysis of the alkene gives octanedioic acid ( suberic acid , suberic acid), which for the production of plasticizers or polyamides is used. The thermolysis of ( Z ) -cyclooctene gives octa-1,7-diene , which is used as a monomer in the rubber and plastics industries. In several further intermediate steps, it is also used to produce various fragrances.
( Z ) -Cyclooctene is a popular substrate for epoxidation. These are usually very selective because the energetically most favorable conformation of ( Z ) -cyclooctene makes the formation of allylic by-products significantly more difficult.
( E ) -Cyclooctene derivatives are used as dienophiles in the bioorthogonal tetrazine ligation.
safety instructions
The vapors of cyclooctene can form an explosive mixture with air when heated above its flash point (24 ° C). This is possible even at higher ambient temperatures.
literature
- Arthur C. Cope, Robert D. Bach: Cyclooctene In: Organic Syntheses . 49, 1969, p. 39, doi : 10.15227 / orgsyn.049.0039 ; Coll. Vol. 5, 1973, p. 315 ( PDF ).
Individual evidence
- ↑ data sheet cis-cyclooctene from Acros, accessed on February 12, 2010.
- ↑ a b c d e f g h Entry on cyclooctene in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Cyclooctene data sheet from Sigma-Aldrich , accessed on May 15, 2017 ( PDF ).
- ^ Louis Fieser , Mary Fieser: Organische Chemie , Verlag Chemie Weinheim, 1982.
- ^ Siegfried Hauptmann , Jürgen Graefe, Horst Remane: Organische Chemie , 1st edition 1976, Deutscher Verlag für Grundstoffindustrie, Leipzig, (2nd revised edition 1980, 3rd edition 1991, Wiley-VCH) ISBN 3-527-30925-X .
- ↑ Maksim Royzen, Glenn PA Yap, and Joseph M. Fox: A Photochemical Synthesis of Functionalized trans Cyclooctenes Driven by metal complexation . In: J. AM. CHEM. SOC. 2008, 130, 3760-3761, doi : 10.1021 / ja8001919 .
- ^ Ulrich Neuenschwander, Ive Hermans: The Conformations of Cyclooctene: Consequences for Epoxidation Chemistry. In: The Journal of Organic Chemistry. 76, 2011, pp. 10236-10240, doi : 10.1021 / jo202176j .