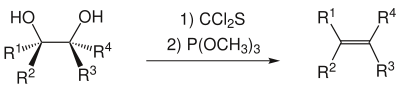Corey Winter Elimination
The Corey-Winter-Elimination , also called Corey-Winter-Fragmentation , is a reaction from the field of organic chemistry . It is used for the synthesis of alkenes from 1,2- diols . In addition to the diol, thiophosgene and trimethyl phosphite are required for this. The reaction is named after its discoverers, Nobel Prize winner Elias James Corey Jr. and Estonian-American chemist Roland Arthur Edwin Winter . The exit groups are not listed in the following overview .
mechanism
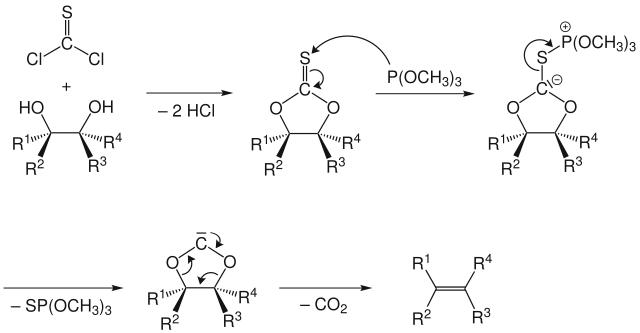
The two diol oxygen atoms gradually attack the thiocarbonyl carbon with a nucleophile . With elimination of hydrogen chloride , a cyclic thio carbonate is formed . In the next step, trimethyl phosphite attacks the sulfur with a nucleophile , forming a carbanion . Cleavage of the thiophosphoric acid ester gives a cyclic carbene . This then breaks down to form the desired alkene with elimination of CO 2 .
The elimination proceeds selectively syn in this reaction . This means that during the first reaction step to form the five-membered ring, a rotation around the carbon-carbon bond in the starting material must take place until the two OH groups are syn or eclipsed to one another. The substituents R 1 to R 4 (see figure) are then either on the same side or on opposite sides of the five-membered ring, depending on their arrangement in the starting material. Whether an ( E ) or ( Z ) alkene is formed must then be determined according to the known rules . For example, trans- 1,2-cyclooctanediol gives ( E ) -cyclooctene . Thiocarbonyl-imidazole , which is easier to handle, is often used instead of thiophosgene , but this worsens the atom economy .
Individual evidence
- ^ EJ Corey, Roland AE Winter: A New, Stereospecific Olefin Synthesis from 1,2-Diols. In: Journal of the American Chemical Society. 85, No. 17, 1963, pp. 2677-2678, doi : 10.1021 / ja00900a043 .
- ^ EJ Corey, B. Hopkins: A mild procedure for the conversion of 1,2-diols to olefins. In: Tetrahedron Letters. 23, No. 19, 1982, pp. 1979-1982, doi : 10.1016 / S0040-4039 (00) 87238-X .
- ↑ Eric Block: Olefin Synthesis by Deoxygenation of Vicinal Diols. In: Organic Reactions. 30, No. 2, 1984, pp. 457-566 ( doi : 10.1002 / 0471264180.or030.02 ).
- ↑ Derek Horton, Charles G. Tindall: Synthesis and reactions of unsaturated sugars. XI. Evidence for a carbenoid intermediate in the Corey-Winter alkene synthesis. In: The Journal of Organic Chemistry. 35, No. 10, 1970, pp. 3558-3559, doi : 10.1021 / jo00835a082 .
Web links
- organic-chemie.ch: Corey-Winter-Olefin-Synthesis