Photosensitizer (chemistry)
The photosensitizer is a substance whose absorption range lies exactly in the wavelength range of the light irradiated with a photo lamp (mostly in the UV range of the absorption spectrum ) and can act as a photochemical " catalyst ". It transfers the light energy to a second molecule that has different absorption properties, but can react after the light energy has been transferred through the sensitizer. This is because only those light rays can be photochemically effective which are absorbed by the substance to be photochemically converted. A naturally occurring sensitizer is, for example, chlorophyll , which plays a role in the light reaction of photosynthesis in photosystems I and II. Sensitizers can be used in drug synthesis for the production of enantiomerically pure compounds and in the development of new methods in oncology to support photodynamic therapy, e.g. B. in skin cancer. In principle, sensitizers can be divided into energy transfer and electron transfer sensitizers.
Energy transfer sensitizers
Energy transfer via the singlet energy state
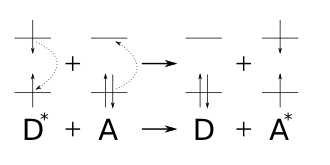
Substances which have their absorption range in the range of the irradiated wavelength are preferably aromatic compounds. These are stimulated photochemically and reach a higher energy state , the S 2 state. From there, the molecule falls into an energetically lower energy state S 1 , a radiationless deactivation. After that, energy is transferred from the lowest energy level of the sensitizer to the highest energy level of the substrate. The substrate then has the energy necessary for a photochemical reaction to take place, from which the target molecule is formed, which would not arise without the addition of a sensitizer. This increases the range of possible uses for photochemically induced reactions. The sensitizer itself does not change its structure. Energy transfer sensitizers are named after their reaction mechanism in which a donor D (the sensitizer) transfers its energy to an acceptor A (the substrate), also known as Förster resonance energy transfer . The energy transfer can take place either via singlet or triplet energy states . The Kasha rule applies here .
Energy transfer via the triplet energy state
During energy transfer, the sensitizer enters the S 1 energy state through light absorption . Then there is the intersystem crossing , a radiationless energy transition to the triplet energy state. The subsequent energy transfer takes place with the release of energy from the triplet state T 1 of the sensitizer to the substrate, which is also in the triplet state. The Jablonski term scheme provides a clear overview of this .
| Sensitizer | Triplet energy | Singlet energy |
|---|---|---|
| Benzophenone | 287 | 319 |
| naphthalene | 250 | 385 |
| Eosin | 178 | 230 |
| Bengal pink | 65 | 214 |
| Porphyrin | ||
| Chlorophyll |
Electron transfer sensitizers
When sensitizing with electron transfer, an electron is transferred from the substrate to the photochemically induced (excited) sensitizer. Sensitizers with good acceptor properties, i.e. high reduction potential, are suitable for this. This means that here too you need an aromatic system that can absorb the incident wavelength, but at the same time it should have a reducing effect after photochemical excitation. This is the case with aromatics which have electronegative substituents (for example CN). The sensitizer absorbs light, reaches a higher energy state and is able to absorb an electron from the substrate. This electron transfer results in a radical ion pair, whereby the sensitizer (in this case the acceptor (A)) becomes the radical anion and the substrate (in this case the donor (D)) becomes the radical cation. The photochemical excitation creates an electron gap in the sensitizer (acceptor) so that the uptake of an electron is facilitated and thus the formation of a radical anion. At the same time, the electron donor becomes a radical cation. Depending on the polarity of the solvent, these radical ion pairs are solvated to different degrees, i.e. they are surrounded by a solvent shell. The resulting photochemical reaction results in different product ratios than with energy transfer sensitizers.
Examples of sensitized photoreactions
- cis - trans isomerization of 1,2-diphenylcyclopropane
- Dimerization of 1,3-cyclohexadiene
- cis - trans isomerization of cyclooctene:
- Isomerization of a cinnamic acid derivative:
- The diastereoselective E / Z isomerization takes place with the addition of (-) - riboflavin as a sensitizer.
Chiral Sensitizers
A current research area is the development of chiral sensitizers, which make it possible to carry out enantiodifferentiating photoreactions. Molecules with an axial center of chirality such as substituted binaphthyls are suitable for this purpose. Substituted triptycenes or molecules with helical chirality , such as pentahelicenedinitrile, could also be used as sensitizers.
A well-known example of a chiral electron transfer sensitizer is 2,2'-dicyano-1,1'-binaphthyl .
literature
- HGO Becker: Introduction to Photochemistry . Deutscher Verlag der Wissenschaften, 1991, ISBN 3-326-00604-7 .
- NJ Turro: Modern Molecular Photochemistry . Menlo Park, 1978, pp. 296-358.
- A. Gilbert, J. Baggott: Essentials of Molecular Photochemistry . Oxford Blackwill Scientific Publications, 1991, ISBN 0-632-02429-1 .
- M. Klessinger , J. Michl: Light absorption and photochemistry of organic molecules . Weinheim, New York, 1989.
- M. Vondenhof, Investigations into Chiral Discrimination in Photoreactions . Dissertation, RWTH Aachen, 1990.
- Martin Vondenhof, Jochen Mattay : Radical ions and photochemical charge transfer phenomena, 28. 1,1'-Binaphthalene-2,2'-dicarbonitrile in photochemically sensitized enantiodifferentiating isomerizations. In: Chemical Reports . 123, No. 12, 1990, pp. 2457-2459, doi: 10.1002 / cber.19901231232 .
- Ji In Kim, Gary B. Schuster: Enantioselective catalysis of the triplex Diels-Alder reaction: a study of scope and mechanism. In: Journal of the American Chemical Society . 114, No. 24, 1992, pp. 9309-9317, doi: 10.1021 / ja00050a011 .
- Mark M. Maturi, Thorsten Bach: Enantioselective Catalysis of the Intermolecular [2 + 2] Photocycloaddition between 2-Pyridones and Acetylenedicarboxylates. In: Angewandte Chemie International Edition . 53, No. 29, 2014, pp. 7661–7664, doi: 10.1002 / anie.201403885 .
- G. Fukuhara, T. Mori, T. Wada and Y. Inoue: The first supramolecular photosensitization of enantiodifferentiating bimolecular reaction: anti-Markovnikov photoaddition of methanol to 1,1-diphenylpropene sensitized by modified β-cyclodextrin In. Chem. Commun. 2006, pp. 1712-1714. doi: 10.1039 / B601674J .
- A. Seeber: Synthesis of new electron transfer and energy transfer sensitizers and their application in photoreactions , dissertation 1995.
- Huanhuan Fan, Guobei Yan, Zilong Zhao, Xiaoxiao Hu, Wenhan Zhang, Hui Liu, Xiaoyi Fu, Ting Fu, Xiao-Bing Zhang and Weihong Tan: A Smart Photosensitizer – Manganese Dioxide Nanosystem for Enhanced Photodynamic Therapy by Reducing Glutathione Levels in Cancer Cells , Angewandte Chemie , 2016, 128, pp. 1-7, doi: 10.1002 / ange.201510748 .
Individual evidence
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 91st – 100th, improved and greatly expanded edition. Walter de Gruyter, Berlin 1985, ISBN 3-11-007511-3 , p. 1020.
- ^ W. Nultsch: Allgemeine Botanik , 1986, pp. 253-263. ISBN 3-13-383308-1 .
- ↑ Willem A. Velema Victor Szymanski, Ben L. Feringa: Photopharmacology: Beyond proof of principle. In: Journal of the American Chemical Society . 136, No. 6, 2014, pp. 2178-2191, doi: 10.1021 / ja413063e .
- ^ Jochen Mattay, Martin Vondenhof: Contact and Solvent-Separated Radical Ion Pairs in Organic Photochemistry. In: Jochen Mattay (Ed.): Photoinduced Electron Transfer III. (= Topics in Current Chemistry. Vol. 159). Springer, Berlin / Heidelberg 1991, ISBN 978-3-540-53257-6 , pp. 219-255.
- ↑ Thorsten Bach: Trend reports: Organic Chemistry 2015 . In: Nachrichten aus der Chemie 64, March 2016, p. 277.
- ^ Jan B. Metternich, Ryan Gilmour: A Bio-Inspired, Catalytic E → Z Isomerization of Activated Olefins . In: Journal of the American Chemical Society . tape 137 , no. 35 , 2015, p. 11254-11257 , doi : 10.1021 / jacs.5b07136 .
- ↑ Helmut Görner, Christian Stammel, Jochen Mattay: Excited state behavior of pentahelicene dinitriles . In: Journal of Photochemistry and Photobiology A: Chemistry . tape 120 , no. 3 , February 1999, p. 171-179 , doi : 10.1016 / S1010-6030 (98) 00423-7 .




![{\ mathrm {D + A + h \ nu \ longrightarrow D + A ^ {{*}} \ longrightarrow \ [D {\ dotsm} A] ^ {*} {\ xrightarrow \} [D ^ {{{\ dot +}}} {\ dotsm} A ^ {{{\ dot -}}}] {\ xrightarrow \} D ^ {{{\ dot +}}} + A ^ {{{\ dot -}}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/dc4c018166f4824d6d089843d6ceecdeded74318)

