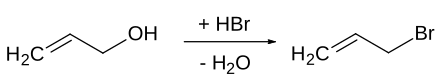3-bromopropene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 3-bromopropene | |||||||||||||||
| other names |
Allyl bromide |
|||||||||||||||
| Molecular formula | C 3 H 5 Br | |||||||||||||||
| Brief description |
colorless, malodorous liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 120.99 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.40 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−119.4 ° C |
|||||||||||||||
| boiling point |
70 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
poorly soluble in water |
|||||||||||||||
| Refractive index |
1.4697 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
12.2 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
3-bromopropene (common name : allyl bromide ) is an organic-chemical compound from the group of alkyl halides .
Extraction and presentation
The synthesis of 3-bromopropene is possible on a laboratory and industrial scale in an S N 1 'reaction from allyl alcohol and hydrobromic acid . The OH group of the allyl alcohol is first protonated and, with the elimination of water, an allyl cation is formed , which then reacts with a bromide ion to form the product. The product can be isolated by extraction and purified by distillation .
Alternatively, the compound can also be prepared via a halogen exchange reaction from allyl chloride and hydrobromic acid or sodium bromide in the presence of a copper and phase transfer catalyst.
properties
3-bromopropene is a colorless, slightly oily liquid with a characteristic sweetish, slightly pungent odor. In moist air it hydrolyzes very slowly to form hydrogen bromide and allyl alcohol. As an alkylating agent, it can cause lasting damage to the body and is therefore classified as toxic.
Safety-related parameters
3-bromopropene forms highly flammable vapor-air mixtures. The compound has a flash point of −1 ° C. The explosion range is between 4.3% by volume (215 g / m 3 ) as the lower explosion limit (LEL) and 7.3% by volume (370 g / m 3 ) as the upper explosion limit (UEL). The ignition temperature is 295 ° C. The substance therefore falls into temperature class T3.
use
In synthetic organic chemistry, 3-bromopropene is mostly used to introduce the allyl group, which is a frequently used protective group for alcohols and amines . The compound is a starting material in the synthesis of drugs such as methoxohexytal , nedocromil , secobarbital and thiamylal .
Individual evidence
- ↑ a b c d e f g h i j k l m n o Entry on allyl bromide in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-68.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-23.
- ↑ a b c Yoffe, D .; Frim, R .; Ukeles, SD; Dagani, MJ; Barda, HJ; Benya, TJ; Sanders, DC: Bromine Compounds , in: Ullmanns Enzyklopädie der Technischen Chemie , Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2013; doi : 10.1002 / 14356007.a04_405.pub2 .
- ↑ a b Entry on Allyl Bromide in the Hazardous Substances Data Bank , accessed July 23, 2015.
- ↑ TSS Dikshith: Handbook of Chemicals and Safety . CRC Press, 2010, ISBN 978-1-4398-2061-2 , pp. 65 ( limited preview in Google Book search).
- ^ A b c E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.