Homocysteine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Structural formula of L- homocysteine | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | L homocysteine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 9 NO 2 S | ||||||||||||||||||
| Brief description |
colorless crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 135.18 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
233 ° C (racemate) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
L- homocysteine ( Hcy ) is a naturally occurring but non-proteinogenic α- amino acid . It isan intermediate product of the one-carbon transferin the metabolism and is formed by S - demethylation of L - methionine as a methyl donor. Raised blood levels for homocysteine can damage the blood vessels . It is also closely related to depression and dementia in old age . Normal laboratory values for blood tests are between 5 and 10 µmol·l −1 . To regulate the homocysteine level in the blood , an adequate supply of betaine and the vitamins B 12 , B 6 and methyl tetrahydrofolate is required. A therapy with folic acid for disease prevention is scientifically controversial.
Two molecules of homocysteine can combine to form homocystine via a disulfide bridge . When the level of homocysteine in the blood increases, homocystine is excreted in the urine ( homocystinuria ).
history
Homocysteine was discovered in 1932 by Vincent du Vigneaud while working on sulfur-containing compounds. But it wasn't until 1962 that Carson and Neil recognized the link between homocysteine and certain diseases. They found significantly increased homocysteine levels in the urine of a group of children with intellectual disabilities and postulated an enzyme defect - classic homocystinuria , caused by a defect in cystathionine β- transferase.
properties
Chemical properties
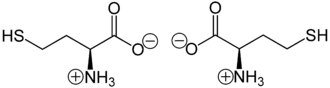
Homocysteine is mainly present as an “inner salt” or zwitterion , the formation of which can be explained by the fact that the proton of the carboxy group migrates to the lone pair of electrons on the nitrogen atom of the amino group .
Zwitterions of L -homocysteine (left) or D -homocysteine (right)
The zwitterion does not migrate in the electric field because it is uncharged as a whole. Strictly speaking, this is the case at the isoelectric point (at a certain pH value), at which the homocysteine also has its lowest solubility in water. Due to its additional CH 2 group compared to cysteine , homocysteine can form a five-membered heterocyclic ring, a so-called thiolactone . This cyclization reaction prevents the formation of stable peptide bonds . A protein that contains homocysteine has a tendency to break down.
Biochemical significance
From L- homocysteine and methyl- FH 4 , the amino acid L - methionine can be formed in a remethylation by the enzyme methionine synthase . Methionine synthase requires vitamin B 12 as a coenzyme . Alternatively, homocysteine can be converted to methionine in the liver and kidneys via betaine homocysteine methyltransferase . The coenzyme for this reaction is betaine. Homocysteine is broken down via transsulfurization, a step that is purely vitamin B 6 dependent.
L- methionine is used biochemically on the one hand for protein synthesis and on the other hand for the formation of S- adenosylmethionine (SAM). SAM is the most important donor for methyl groups in cellular metabolism. Once it has given up its methyl group, S- adenosylhomocysteine (SAH) is formed, which is hydrolyzed to adenosine and L -homocysteine:
SAH inhibits methylation reactions, so its breakdown to homocysteine is absolutely necessary in order to be able to maintain methylation reactions. If the breakdown of homocysteine is disturbed, the important methylation reactions in the cell are also disturbed.
Importance to health
Homocysteine occurs naturally in the human body. However, increased values lead to the clinical picture of hyperhomocysteinemia . Even moderately high homocysteine levels can also increase the risk of cardiovascular diseases . Lowering homocysteine levels could have a preventative effect, but there are no studies to support this.
Elevated homocysteine levels are also associated with eye diseases, particularly retinopathy , pseudoexfoliation syndrome , cataract , optic atrophy and arteriosclerosis of the retinal vessels; whether there is also a connection to maculopathy is controversial.
In the case of a vitamin B12 deficiency , homocysteine is increased and can be used as a progress parameter for the therapy of a vitamin B12 deficiency (if the therapy is effective, the increased homocysteine levels should return to normal).
literature
- Olaf Stanger: Homocysteine: Basics, Clinic, Therapy, Prevention . Maudrich, Vienna / Munich / Bern 2004, ISBN 3-85175-766-1 .
- Jeremy M. Berg, John L. Tymoczko, Lubert Stryer: Biochemistry. 5th edition. Spectrum Akademischer Verlag, Heidelberg 2003, ISBN 3-8274-1303-6 .
- Per Magne Ueland, Helga Refsum, Lars Brattström: 8. Plasma Homocysteine and Cardiovascular Disease . In: Robert Francis (Ed.): Atherosclerotic cardiovascular disease, hemostasis, and endothelial function . M. Dekker, New York 1992, ISBN 0-8247-8726-9 , pp. 183–236 ( PDF 2.9 MB ( memento of November 20, 2004 in the Internet Archive )).
- Wolfgang Herrmann, Rima Obeid: Vitamins in the prevention of human diseases. 2011, ISBN 978-3-11-021448-2 .
- Wolfgang Herrmann, Rima Obeid: The obligatory folic acid fortification of food: A controversial topic in Germany. The Mandatory Fortification of Staple Foods with Folic Acid: A Current Controversy in Germany. Ärzteblatt review article, doi: 10.3238 / arztebl.2011.0249
Individual evidence
- ↑ a b entry on homocysteine. In: Römpp Online . Georg Thieme Verlag, accessed on June 1, 2014.
- ↑ a b data sheet DL-Homocysteine from Sigma-Aldrich , accessed on November 5, 2016 ( PDF ).
- ↑ German Institute for Nutritional Research Potsdam-Rehbrücke: Combination of low vitamin B12 and low folate levels increases the risk of stroke. Press release of September 25, 2007, accessed February 9, 2013.
- ↑ Cornelia Weikert et al: B Vitamin Plasma Levels and the Risk of Ischemic Stroke and Transient Ischemic Attack in a German Cohort. In: Stroke. 38, 2007, pp. 2912-2918. doi: 10.1161 / STROKEAHA.107.486068 .
- ^ D. Gruson: Cardiovascular diseases and homocysteine, a short summary of a long story. In: J Int Clin Chem. 14, 2003, p. 3.
- ↑ Vincent du Vigneaud: A Trail of Sulfa Research: From Insulin to Oxytocin. In: Nob Lec. 1955, pp. 1-10. (PDF)
- ^ NA Carson, DW Neill: Metabolic abnormalities detected in a survey of mentally backward individuals in Northern Ireland . In: Arch. Dis. Child. tape 37 , October 1962, p. 505-513 , doi : 10.1136 / adc.37.195.505 , PMID 14018926 , PMC 2012909 (free full text).
- ↑ HS Baernstein: In: Journal of Biological Chemistry. 106, 1934, p. 451.
- ↑ P. Durand, M. Prost, N. Loreau: Impaired homocysteine metabolism and atherothrombotic disease. In: Lab Invest . 81, 2001, pp. 645-672. PMID 11351038 .
- ^ German Green Cross, accessed on November 25, 2015
- ↑ TA Ajith, Ranimenon: Homocysteine in ocular diseases . In: Clin. Chim. Acta . tape 450 , October 2015, p. 316-21 , doi : 10.1016 / j.cca.2015.09.007 , PMID 26343924 .
- ↑ A. Pinna, F. Zaccheddu, F. Boscia, C. Carru, G. Solinas: Homocysteine and risk of age-related macular degeneration: A systematic review and meta-analysis . In: Acta Ophthalmol . tape 96 , no. 3 , May 2018, p. e269 – e276 , doi : 10.1111 / aos.13343 , PMID 27966830 .
- ^ S. Rajan, JI Wallace, SA Beresford, KI Brodkin, RA Allen, SP Stabler: Screening for cobalamin deficiency in geriatric outpatients: prevalence and influence of synthetic cobalamin intake. In: J Am Geriatr Soc. 50 (4), 2002, pp. 624-630. PMID 11982661 .
- ↑ Wolfgang Herrmann, Rima Obeid: Causes and early diagnosis of vitamin B12 deficiency . In: Deutsches Ärzteblatt . tape 105 , no. 40 , 2008, p. 680–685 , doi : 10.3238 / arztebl.2008.0680 ( online ).