Leucine
As Leucine firstly summarizes the four isomeric amino acids leucine , isoleucine , tert -leucine and norleucine together. In comparison with the four butanols , they can be viewed as butyl- substituted glycines; thus all four variants are represented. The number of the maximum possible aliphatic amino acids with n carbon atoms in the side chain (without taking the stereoisomers into account) is equal to the number of ternary tree graphs with n nodes. This results in the number sequence 1 (for n = 0), 1, 1, 2, 4, 8, 17, ... For n = 4 there are 4 possibilities. These are realized by the amino acids mentioned above.
Leucine and isoleucine belong to the proteinogenic amino acids , i. That is, they are building blocks of the proteins of living things and are encoded by the genetic code .
If the stereoisomerism is also taken into account, 6 more isomers must be added: (a) D -leucine, (b) D -isoleucine, (c) L - allo -isoleucine, (d) D - allo -isoleucine, (e ) D - tert -leucine and (f) D -norleucine. They are each described under the associated amino acid articles.
| Leucine | ||||
| Surname | L - leucine | L - isoleucine | L - tert -leucine (terleucine) | L - norleucine |
| other names | 2-amino-4-methyl pentanoic acid, iso -Butylglycin |
2-amino-3-methylpentanoic acid, sec -butylglycine |
2-amino-3,3-dimethylbutanoic acid, tert -butylglycine |
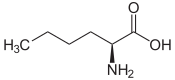
2-aminohexanoic acid, n -butylglycine |
| Structural formula | 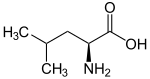 |
 |
 |
|
| CAS number | 61-90-5 | 73-32-5 | 20859-02-3 | 327-57-1 |
| PubChem | 6106 | 791 | 164608 | 21236 |
| Molecular formula | C 6 H 13 NO 2 | |||
| Molar mass | 131.18 g mol −1 | |||
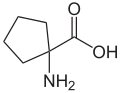
The cycloleucine (1-aminocyclopentane-1-carboxylic acid) can be considered as the norleucine cyclic derivative. It differs from this u. a. by a molar mass lower by two hydrogen atoms (129.16 g mol −1 ). The defining structural element is a cyclopentane ring. The α-carbon atom is also not a stereocenter; So cycloleucine is not chiral.
See also
literature
- Jeremy M. Berg, John L. Tymoczko, Lubert Stryer : Biochemistry. 6 edition, Spektrum Akademischer Verlag, Heidelberg 2007. ISBN 978-3-8274-1800-5 .
- Donald Voet, Judith G. Voet: Biochemistry. 3rd edition, John Wiley & Sons, New York 2004. ISBN 0-471-19350-X .
- Bruce Alberts , Alexander Johnson, Peter Walter, Julian Lewis, Martin Raff, Keith Roberts: Molecular Biology of the Cell , 5th Edition, Taylor & Francis 2007, ISBN 978-0815341062 .
Individual evidence
- ↑ K. Grützmann, S. Böcker, S. Schuster: Combinatorics of aliphatic amino acids , Naturwissenschaften 98 (2011) pp. 79-86 ( doi : 10.1007 / s00114-010-0743-2 ; PMID 21120449 ).