Valinomycin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Valinomycin | |||||||||||||||
| other names |
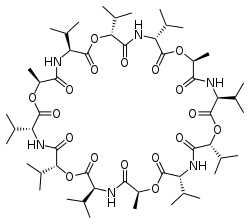
Cyclo ( D -α-hydroxyisovaleryl- D -valyl- L -lactoyl- L -valyl- D -α-hydroxyisovaleryl- D -valyl- L -lactoyl- L -valyl- D -α-hydroxyisovaleryl- D -valyl- L - lactoyl- L -valyl) |
|||||||||||||||
| Molecular formula | C 54 H 90 N 6 O 18 | |||||||||||||||
| Brief description |
beige solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 1111.32 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
190 ° C (decomposition) |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Valinomycin is a Cyclodeka- depsipeptide and is one of the macrolide - antibiotics . It is produced by several types of Streptomycetes (e.g. Streptomyces fulvissimus ).
structure
Valinomycin consists of the enantiomeric amino acids L - valine and D - valine and the hydroxy acids L - lactate and D - hydroxyisovalerate . The cyclic structure, consisting of the triple repetitive tetra-depsipeptide unit [- L -Lac– L -Val– D -Hiv– D -Val–] 3 , forms a symmetrical ring with 36 atoms of alternating amide and ester bonds .
properties
Valinomycin is an ionophore that selectively transports potassium ions. Here, K + is complexed in a cage-like structure and transported through the cell membrane. As a result of these transport processes, the membrane potential breaks down and the cell dies.
The complex formation constant for the potassium-valinomycin complex is 10 6 , while it is only 10 for the sodium-valinomycin complex. This great difference (selectivity) is of great importance for the transport processes in biological systems.
use
Most electrodes for the detection of potassium ions use the specific complexation of potassium ions by valinomycin, which is embedded in a plastic membrane in a concentration of around 0.7%.
Web links
- The Virtual Museum of Minerals and Molecules: Valinomycin
credentials
- ↑ Valinomycin data sheet from Sigma-Aldrich , accessed on April 25, 2011 ( PDF ).
- ↑ a b Entry on valinomycin in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ Entry on valinomycin. In: Römpp Online . Georg Thieme Verlag, accessed on December 29, 2014.
- ↑ a b Entry on valinomycin in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ Rose Lars and Jenkins ATA: The effect of the ionophore valinomycin on biomimetic solid supported lipid DPPTE / EPC membranes . In: Bioelectrochem. . 70, No. 2, 2007, pp. 387-393. PMID 16875886
- ^ Cammann K: Ion-selective bulk membranes as models . In: Top. Curr. Chem. . 128, 1985, pp. 219-258.
- ↑ Safiulina D, Veksler V, Zharkovsky A and Kaasik A: Loss of mitochondrial membrane potential is associated with increase in mitochondrial volume: physiological role in neurones . In: J Cell Physiol . 206, No. 2, 2006, pp. 347-353. PMID 16110491
