Amoxicillin
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
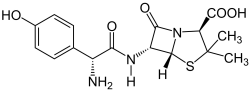
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Amoxicillin | ||||||||||||
| other names |
(2 S , 5 R , 6 R ) -6 - [( R ) -2-Amino-2- (4-hydroxyphenyl) acetamido] -3,3-dimethyl-7-oxo-4-thia-1-azabicyclo [ 3.2.0] heptane-2-carboxylic acid |
||||||||||||
| Molecular formula | C 16 H 19 N 3 O 5 S | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| properties | |||||||||||||
| Molar mass | 365.40 g mol −1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Amoxicillin is a broad spectrum antibiotic from the group of aminopenicillins and thus belongs to the group of active substances known as β-lactam antibiotics . The drug has been approved for the treatment of infections since 1981 and can be used orally or parenterally . Finished medicinal products are sold under different names .
application
Amoxicillin is used against infections of the gastrointestinal tract , the biliary tract and the lower urinary tract , against respiratory tract infections , rhinosinusitis and infections of the middle ear and sometimes against skin infections ( e.g. after animal bites ). It is effective against gram-positive and some gram-negative bacteria, such as enterobacteria , but does less damage to the intestinal flora than ampicillin .
Due to its acid stability, amoxicillin is effective orally ; common forms of administration are dry juice or tablets. Amoxicillin can also be injected.
The spectrum of activity can be expanded by combining it with a β-lactamase inhibitor such as clavulanic acid . β-lactamase is an enzyme produced by some bacteria that inactivates antibiotics such as amoxicillin that are not stable to β-lactamase . Clavulanic acid protects amoxicillin from being broken down by most of the β-lactamases in staphylococci .
The plasma half-life averages around 60 minutes in healthy kidneys. Amoxicillin is for the most part excreted via the kidneys, a small proportion in the bile .
Side effects
As with all penicillins , amoxicillin can often cause side effects in the form of an allergic reaction ( exanthema , urticaria ) of varying severity, and rarely up to anaphylactic shock and exfoliative skin reactions (amoxicillin is contraindicated in the case of known penicillin allergy). Hypersensitivity reactions such as drug fever are more common . A non- urticarial rash can occur (especially in patients with infectious mononucleosis ), which is also not a real penicillin allergy and should be inspected by the dermatologist. Inflammation of the mucous membranes and an increase in liver enzyme levels are also common. In addition, diarrhea, nausea and vomiting are very common. Penicillins can usually also be prescribed during pregnancy . Symptoms of fatigue, sleep disorders and mild states of confusion occur rarely. Amoxicillin very rarely causes pseudomembranous colitis , changes in the blood count ( eosinophilia , neutropenia , thrombocytopenia , leukopenia ) or liver dysfunction.
synthesis
Starting from the carboxylate ( 1 ) - a derivative of ( R ) -4-hydroxyphenylglycine - amoxicillin ( 4 ) can be synthesized as follows:
In the first step, 1 is reacted with a carboxylic acid chloride with the addition of a base (e.g. 4-methylmorpholine ), the mixed carboxylic acid anhydride ( 2 ) being formed in a nucleophilic substitution reaction . Then 2 is used to acylate 6-aminopenicillanic acid ( 3 ), whereby amoxicillin ( 4 ) is formed after splitting off the protective group , which can be isolated as a yellowish white powder.
Trade names
Monopreparations
Human medicinal products: Amoxibeta (D), Amoxilan (A), Amoxypen (D), Azillin (CH), Baktocillin (D), Clamoxyl (A, CH), Infectomox (D), Jutamox (D), Ospamox (A), Spectroxyl (CH), Supramox (CH)
Veterinary medicines: Aciphen (D), Amox (D), Amoxanil (D), Amoxin (D), Amoxisel (D), Amoxival (D), Amoxy (D), Belamox (D), Bioamoxi (D), Clamoxyl (D ), Duphamox (D), Hostamox (D), Klatocillin (D), Octacillin (D), Parkemoxin (D), Tamox (D), Vetrimoxin (D), Veyxyl (D), Wedemox (D)
Combination preparations
- With clavulanic acid : Human medicines: Amoclav (D), AmoclanHexal (A), Amoxacid (A), AmoxiPLUS ratiopharm (A), Amoxi-saar plus (D), Amoxicomp (A), Augmentan (D), Augmentin (A, CH, D), Benomox (A), Betamoclav (A), Clavamox (A), Clavex (A), Clavolek (A), Clavoplus (A), Co-Amoxiclav (A), Curam (A), InfectoSupramox (D), Lekamoxiclav (A), Xiclav (A)
Veterinary medicinal products: Amoxiclav (D), Clavaseptin (D), Kesium (D), Nicilan (D), Synulox (D), Amoxi-Clavulan (D)
- With flucloxacillin : Flanamox (D)
- With pantoprazole and clarithromycin : Zacpac (D)
There are also other generics for both mono- and combi-preparations.
Individual evidence
- ↑ a b c Amoxicillin data sheet from Sigma-Aldrich , accessed on March 9, 2011 ( PDF ).
- ↑ See Marianne Abele-Horn: Antimicrobial Therapy. Decision support for the treatment and prophylaxis of infectious diseases. With the collaboration of Werner Heinz, Hartwig Klinker, Johann Schurz and August Stich, 2nd, revised and expanded edition. Peter Wiehl, Marburg 2009, ISBN 978-3-927219-14-4 , p. 338.
- ^ A b c Axel Kleemann , Jürgen Engel, Bernhard Kutscher, Dietmar Reichert: Pharmaceutical Substances - Syntheses, Patents and Applications of the most relevant APIs. 5th edition. Georg Thieme Verlag, 2009, ISBN 978-3-13-558405-8 , p. 69.

![Overview of the synthesis of amoxicillin [3]](https://upload.wikimedia.org/wikipedia/commons/thumb/1/1d/Amoxicillin_Sythesis_V4.svg/440px-Amoxicillin_Sythesis_V4.svg.png)