4-methylmorpholine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
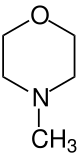
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 4-methylmorpholine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 11 NO | |||||||||||||||
| Brief description |
colorless liquid with an amine-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 101.15 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.91 g cm −3 |
|||||||||||||||
| Melting point |
−66 ° C |
|||||||||||||||
| boiling point |
116 ° C |
|||||||||||||||
| Vapor pressure |
30 hPa (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.4355 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
4-methylmorpholine is a chemical compound from the group of nitrogen - oxygen - heterocycles .
Extraction and presentation
4-methylmorpholine can be obtained by reacting methylamine with diethylene glycol and by hydrogenolysis of N -formylmorpholine . The compound can also be obtained from morpholine and formaldehyde or methanol .
properties
4-methylmorpholine is a highly flammable, volatile, colorless liquid with an amine-like odor that is miscible with water. Its aqueous solution has an alkaline reaction.
use
4-methylmorpholine is used as a solvent for dyes, resins, waxes and medicines. It serves as a crosslinker in the production of polyurethane foams , elastomers and adhesives. It is used as a precursor for the production of N -methylmorpholine- N -oxide - and morpholinium cations -based ionic liquids. It is used as a corrosion inhibitor and anti-limescale in industry. It is also used in paint removers.
safety instructions
The vapors of 4-methylmorpholine can form an explosive mixture with air ( flash point 13 ° C, ignition temperature 165 ° C).
Individual evidence
- ↑ a b c d e f g h i j k l Entry on 4-methylmorpholine in the GESTIS substance database of the IFA , accessed on December 3, 2018(JavaScript required) .
- ↑ a b c Entry on 4-methylmorpholine. In: Römpp Online . Georg Thieme Verlag, accessed on December 3, 2018.
- ↑ a b Data sheet 4-Methylmorpholine, 99% from AlfaAesar, accessed on December 3, 2018 ( PDF )(JavaScript required) .
- ↑ Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke (2005). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi : 10.1002 / 14356007.a02_001


