Degarelix
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
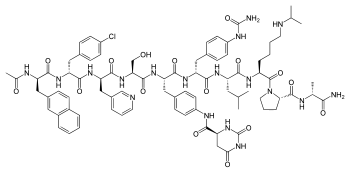
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Degarelix | |||||||||||||||||||||
| Molecular formula | C 82 H 103 ClN 18 O 16 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 1692.31 g mol −1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Degarelix is a synthetic peptide that is used to treat advanced hormone-dependent prostate cancer . It is a so-called GnRH antagonist that brings about a rapid reduction in testosterone levels within a few days and thus achieves an immediate effect similar to an orchiectomy . In this context, one speaks of androgen deprivation therapy (ADT). The primary alternative to the GnRH antagonist, the LHRH agonists, require four weeks to lower the sex hormone levels equally.
The drug was approved in the US since December 2008. In February 2009, the granted European Medicines Agency the company Ferring Pharmaceuticals , the marketing authorization for degarelix (trade name FIRMAGON) throughout the European Union.
Mechanism of action
Degarelix is based on the body's own gonadotropin-releasing hormone (GnRH) and acts as a GnRH antagonist. The active ingredient blocks the receptors in the anterior lobe of the pituitary gland directly and thus leads to a rapid, significant and long-lasting decrease in testosterone levels within a few days. An initial increase in testosterone as with the LHRH agonists does not occur here.
After the administration of degarelix, the testosterone level was reduced below the castration level in 99% of the patients.
Study results
Epidemiological data have shown that androgen deprivation therapy leads to changes in metabolism (reduced glucose tolerance or worsening of existing diabetes mellitus ) and that there may be an increased risk of cardiovascular diseases. Studies have shown that a GnRH antagonist could reduce the risk of cardiovascular events during androgen deprivation therapy by around 50% compared to LHRH agonists. Since about 33% of all patients with hormone-sensitive prostate cancer already have cardiovascular risks and / or diseases before treatment, treatment with a GnRH antagonist is suitable for this risk group.
Prostate carcinoma metastasize primarily to the local lymph nodes or the skeleton ( bone metastases ). Testosterone promotes the growth of cancer cells and thus the growth in size of the tumor and metastases .
A quick and direct lowering of the testosterone level can have a positive effect on the pain associated with bone metastases. Studies have shown that degarelix is 50% more likely to be pain-free than LHRH agonists (goserelin).
Side effects
Side effects can occur with ADT with degarelix . Many of these are directly linked to the rapid drop in testosterone levels and represent typical reactions of the body to hormone withdrawal. The occurrence of these side effects also shows that the therapy is working successfully.
Hot flashes and injection site reactions are very common.
Web links
- EUROPEAN PUBLIC ASSESSMENT REPORT (EPAR) for FIRMAGON, as of August 11, 2009 on the website of the European Medicines Agency (EMA) , accessed on February 19, 2016.
- Entries in the NIH study registry
Individual evidence
- ↑ There is not yet a harmonized classification for this substance . A label of [No public or meaningful name is available] in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on January 13, 2020, is reproduced from a self-classification by the distributor .
- ↑ a b Technical information on Firmagon by Ferring, information as of September 2013.
- ↑ Dirk Manski: Antiandrogenic hormone therapy: Degarelix and Abarelix (LHRH antagonists) , on Urologielehrbuch.de.
- ^ PR Newswire: FDA approves Ferring Pharmaceuticals' Degarelix (generic name) for the treatment of advanced prostate cancer. PR Newswire, Europe Ltd 2008, accessed March 2, 2009.
- ↑ Klotz, L., Boccon-Gibod, L., Shore, ND, Andreou, C., Persson, B.-E., Cantor, P., Jensen, J.-K., Olesen, TK, Schröder, FH : The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. In: BJU Int . 102 (2008), pp. 1531-1538.
- ↑ Technical information on Trenantone from Takeda, as of August 2018.
- ↑ Anderson M et al .: Management of advanced prostate cancer: can we improve on androgen deprivation therapy? In: British Association of Urological Surgeons (Ed.): BJU International . No. 101 . Wiley-Blackwell-Verlag, p. 1497-1501 .
- ↑ Lehmann J et al. Influence of cardiovascular (CV) comorbidities on the selection of hormone deprivation therapy (HDT) in the treatment of metastatic prostate cancer (mPCa). Poster presented at the Genitourinary Cancers Symposium, San Francisco, February 8-10, 2018.
- ↑ Albertsen PC et al .: Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. In: European Association of Urology (Ed.): Eur. Urol. tape 65 , no. 3 . Elsevier-Verlag, p. 565-573 .
- ↑ Eisenhardt A et al. Cardiovascular comorbidities in German prostate cancer patients under androgen deprivation therapy. Resuslt from a retrospective analysis of patient data in urological outpatient centers. Poster presented at the Genitourinary Cancers Symposium, San Francisco, February 8-10, 2018.
- ↑ a b Tombal B et al .: Efficacy and safety of a 3-monthly depot of degarelix compared with goserelin in prostate cancer. In: European Urology Supplements . tape 11 , no. 5 , 2012, p. 228 .