Sultamicillin
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
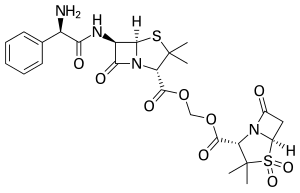
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Sultamicillin | ||||||||||||
| other names |
[(2 R ) -3,3-dimethyl-4,4,7-trioxo-4λ 6 -thia-1-azabicyclo- [3.2.0] heptane-2-carbonyl] oxymethyl- (2 R ) -6- [ (2 S ) -2-amino-2-phenyl-acetyl] amino ( IUPAC ) |
||||||||||||
| Molecular formula | C 25 H 30 N 4 O 9 S 2 | ||||||||||||
| Brief description |
white to almost white, crystalline, slightly hygroscopic powder |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| Mechanism of action |
Inhibition of cell wall synthesis by sensitive bacteria and inactivation of numerous β-lactamases |
||||||||||||
| properties | |||||||||||||
| Molar mass | 594.66 g mol −1 | ||||||||||||
| solubility |
practically insoluble in water, very sparingly soluble in methanol , practically insoluble in ethanol |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Sultamicillin is a prodrug - antibiotic in which the β-lactam antibiotic ampicillin as a bactericidal component and sulbactam as an inhibitor of β-lactamase are chemically linked by an ester bond . This binding increases the absorption via the intestine ( oral bioavailability ) compared to the combination of both substances as free molecules. After absorption has taken place, the bond is split hydrolytically .
Sultamicillin is a broad spectrum antibiotic. It also has an effect on β-lactamase producers (including Staphylococcus aureus ) and anaerobic bacteria. Areas of application are mainly urinary and respiratory infections caused by bacteria sensitive to ampicillin, but also gonorrhea .
The plasma half-life is approximately one hour. Ampicillin and sulbactam are excreted renally unchanged . The main side effects described were diarrhea , allergic reactions are possible.
 |
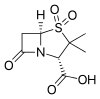
|
| Structural formula of ampicillin | Sulbactam structural formula |

|
|
| Structural formula of sultamicillin, blue = ampicillin, red = sulbactam |
|
Trade names
Unacid PD oral (D), sultamicillin ratio 375 mg (D), Unasyn (D, A)
Individual evidence
- ↑ a b SULTAMICILLIN CRS data sheet (PDF) at EDQM , accessed on March 26, 2009.
- ↑ Infoportal Pfizer
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ JC Frölich, W. Kirch: Practical medicine therapy . Springer-Verlag, 2013, ISBN 978-3-662-09397-9 , pp. 949 ( limited preview in Google Book search).
- ^ Gustav Paumgartner, Gerhard Steinbeck: Therapy for internal diseases . Springer-Verlag, 2013, ISBN 978-3-662-10475-0 , pp. 1617 ( limited preview in Google Book search).
- ↑ a b R. Ho ̈hl: Oral antibiotics in clinic and practice Practice-oriented recommendations for antibiotic therapy of mild and moderate bacterial infections in adults in outpatient and inpatient areas . Springer-Verlag, 2009, ISBN 978-3-642-00522-0 , pp. 16 ( limited preview in Google Book search).
- ^ A b Franz von Bruchhausen: Hager's Handbook of Pharmaceutical Practice . Springer-Verlag, 1930, ISBN 978-3-540-52688-9 , pp. 749 ( limited preview in Google Book search).