Sulbactam
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
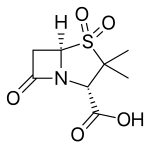
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Sulbactam | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 8 H 11 NO 5 S | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 233,24 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
148–151 ° C or 170 ° C (dec.) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Sulbactam is a synthetic penicillanic acid sulfone which is used as a drug from the group of β-lactamase inhibitors in combination with β-lactam antibiotics (e.g. with ampicillin as ampicillin / sulbactam ).
Clinical information
Application areas (indications)
Sulbactam supports the antibiotic and is not bactericidal or bacteriostatic in clinically relevant terms . Some bacteria form the enzyme β-lactamase , which is able to split the β-lactam ring, which some antibiotics contain in their chemical structure ( penicillin , cephalosporin , carbapenem , monobactam ). The cleavage changes the chemical structure of the antibiotic in such a way that it becomes ineffective.
Dosis, kind and Time of the Use
Sulbactam is given parenterally before the antibiotic, which should have a half-life similar to that of sulbactam. Depending on the sensitivity of the pathogen, the addition of 0.5–1.0 g sulbactam is recommended. The maximum daily dose is 4 g sulbactam.
Contraindications (contraindications)
Sulbactam must not be prescribed if the patient is known to be hypersensitive to β-lactam antibiotics. In children under one year of age, the effect of the drug has not been fully understood and should not be used. Without the simultaneous administration of a β-lactam antibiotic, the use of sulbactam does not make sense, as it does not have any significant antibacterial properties of its own.
No indications of embryotoxic or teratogenic effects were found in animal experiments . However, there is insufficient experience with its use in humans. The drug is excreted in breast milk , and no harm has been reported to the infant. Treatment during pregnancy and breastfeeding may only be carried out according to strict indications.
Drug interactions
Sulbactam and the following drugs are incompatible when used at the same time - precipitation, discoloration and clouding occur - therefore they must be administered separately: Metronidazole ; Procaine 2%; Aminoglycosides ; parenterally applicable tetracycline derivatives such as oxytetracycline , rolitetracycline and doxycycline ; also sodium pentothal ; Prednisolone ; Suxamethonium chloride and norepinephrine .
Special patient groups (diabetics, kidney patients)
Dosage in severe renal impairment
- Cr clearance : 15-30 ml / min; Maximum daily dose: 2 g
- Cr clearance: <15 ml / min; Maximum daily dose: 1 g
Adverse effects (side effects)
- Allergic reactions such as skin reactions, eosinophilia , anaphylactic shock
- Gastrointestinal disorders
- Local reactions
- Interstitial nephritis (rare)
- Increase in liver values as side effects of the combined antibiotics (rare)
- Note the side effects of the combined antibiotic!
Pharmacological properties
Mechanism of action (pharmacodynamics)
Sulbactam inhibits most forms of β-lactamases produced by various bacteria, but not the β-lactamase "ampC cephalosporinase". This particular β-lactamase is often produced by Pseudomonas aeruginosa , Citrobacter , Enterobacter and Serratia . Sulbactam binds irreversibly to the enzyme β-lactamase and thus prevents the successful function of this enzyme, i.e. it prevents the inactivation of the antibiotic, which can thus exert its therapeutic effect on the bacterium.
Absorption and distribution in the body (pharmacokinetics)
Sulbactam is hardly absorbed in the gastrointestinal tract and is therefore generally given parenterally in the form of a short infusion. With an infusion duration of 15 minutes, sulbactam reaches its serum concentration maximum immediately after the end of the infusion.
The intramuscular injection reported an almost complete and reliable absorption, with a bioavailability of 99% about 30-60 minutes after injection. Sulbactam is well distributed in tissues and body fluids (including skin blisters). However, the distribution in the CSF is limited, but increased if there is inflammation.
The plasma protein binding of sulbactam is 38%, so it has the greatest affinity of the β-lactamase inhibitors for serum proteins. It has an approximate plasma half-life of 1 hour.
Mainly sulbactam is eliminated via glomerular filtration and tubular secretion. Metabolism is not known. The elimination takes place mainly renally (80% unchanged excreted substance) and can therefore be reduced by the administration of probenecid .
Manufacturing
A multi-step synthesis for sulbactam is described in the literature.
Trade names
Combactam (D, A), as well as a generic (D)
Ampicillin / Sulbactam (D, A), Ampicillin comp (D), Unacid (D), Unasyn (A)
literature
- Berdel V. et al. [Ed.]; Diehl Classen: Internal Medicine . Urban & Fischer in Elsevier, Munich 2006, ISBN 978-3-437-44405-0 .
- Chemotherapy Journal 1991; 6th
Individual evidence
- ↑ Entry on sulbactam. In: Römpp Online . Georg Thieme Verlag, accessed on April 17, 2011.
- ↑ Registration dossier on sulbactam ( GHS section ) at the European Chemicals Agency (ECHA), accessed on July 12, 2020.
- ↑ a b c Technical information Combactam ® - PFIZER PHARMA GmbH .
- ↑ Noguchi JK, Gill MA. Sulbactam: a beta-lactamase inhibitor. Clin Pharm. 1988 Jan; 7 (1): 37-51, PMID 3278833 .
- ^ Axel Kleemann , Jürgen Engel, Bernd Kutscher and Dietmar Reichert: Pharmaceutical Substances, 4th edition (2000), 2 volumes published by Thieme-Verlag Stuttgart, ISBN 978-1-58890-031-9 ; online since 2003 with biannual additions and updates.