Oxacillin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
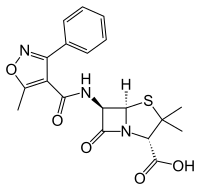
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Oxacillin | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 401.44 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
188 ° C (oxacillin monosodium salt monohydrate) |
|||||||||||||||||||||
| pK s value |
2.72 |
|||||||||||||||||||||
| solubility |
Water: 27.8 mg l −1 (25 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Oxacillin is an antibiotic from the group of β-lactam antibiotics , which belongs to the class of isoxazolyl penicillins. It belongs to the penicillinase- resistant penicillins , the so-called staphylococcal penicillins.
The clinical area of action of oxacillin is penicillinase- producing staphylococci strains. Here it serves as the key antibiotic. Nosocomial infections are, however, increasingly multi-resistant Staphylococcus aureus caused strains, one speaks in this case of O xacillin- R esistenten- S taphylococcus a ureus strains in short ORSA. The more commonly used term as methicillin-resistant Staphylococcus aureus , or MRSA for short, is synonymous. The background to these different names is the approval of methicillin in the USA and oxacillin in Europe.
Other penicillinase-resistant antibiotics are cloxacillin , dicloxacillin , flucloxacillin and methicillin , although methicillin is nowadays used neither therapeutically nor for testing sensitivity.
Trade names
- InfectoStaph (D), Stapenor Salbe ad us.vet. (Cattle) (D))
Individual evidence
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, ISBN 978-0-911910-00-1 , p. 1190.
- ↑ a b c Entry on oxacillin in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b Datasheet Oxacillin sodium salt monohydrate from Sigma-Aldrich , accessed on November 7, 2016 ( PDF ).
- ↑ ABDA database (as of December 10, 2009).
Web links
- Entry on Oxacillin at Vetpharm