Dicloxacillin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Dicloxacillin | |||||||||||||||||||||
| other names |
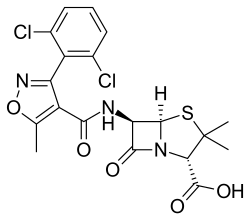
(2 S , 5 R , 6 R ) -6 - {[3- (2,6-dichlorophenyl) -5-methyl-1,2-oxazole-4-carbonyl] amino} -3,3-dimethyl-7- oxo-4-thia-1-azabicyclo [3.2.0] heptane-2-carboxylic acid |
|||||||||||||||||||||
| Molecular formula | C 19 H 17 Cl 2 N 3 O 5 S | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 470.327 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Dicloxacillin is an antibiotic chemical compound from the group of penicillins and is obtained semi-synthetically.
properties
Dicloxacillin is resistant to penicillinase .
use
Dicloxacillin is used in particular against staphylococcal infections and is mostly administered orally. Specifically, it is used for skin and upper respiratory tract infections and as a follow-up treatment for osteomyelitis . It was patented in 1961 and approved for medical use in the United States in 1968. Against methicillin-resistant bacteria such as S. aureus ssp. Combinations of dicloxacillin and amikacin act synergistically. As with other penicillinase-resistant antibiotics, the administration of dicloxacillin can in rare cases lead to severe liver damage. This is why it is only recommended for use on bacteria resistant to penicillinase.
The sodium salt is used in its hydrate form in finished medicinal products.
Environmental aspects
In 2019, various rivers in Europe were examined for their pollution with pesticides and medicines. Dicloxacillin has been detected in about 66% of the rivers.
Individual evidence
- ↑ a b Caymanchem: MSDS Dicloxacillin (sodium salt hydrate) , accessed June 12, 2019.
- ↑ DICLOXACILLIN SODIUM- dicloxacillin sodium capsule. Retrieved June 3, 2019 .
- ↑ Sharon S. Castle: Dicloxacillin . In: xPharm: The Comprehensive Pharmacology Reference . Elsevier, 2007, ISBN 978-0-08-055232-3 , pp. 1-5 , doi : 10.1016 / b978-008055232-3.61589-2 .
- ^ Fischer, János., Ganellin, CR (C. Robin): Analogue-based drug discovery . Wiley-VCH, Weinheim 2006, ISBN 3-527-60749-8 .
- ↑ Guadalupe Miranda-Novales, Blanca E. Leaños-Miranda, Mariano Vilchis-Pérez, Fortino Solórzano-Santos: In vitro activity effects of combinations of cephalothin, dicloxacillin, imipenem, vancomycin and amikacin against methicillin-resistant Staphylococcus spp. strains . In: Annals of Clinical Microbiology and Antimicrobials . tape 5 , October 12, 2006, p. 25 , doi : 10.1186 / 1476-0711-5-25 , PMID 17034644 , PMC 1617116 (free full text).
- ^ R. Olsson, B.-E. Wiholm, C. Sand, L. Zettergren, R. Hultcrantz: Liver damage from flucloxacillin, cloxacillin and dicloxacillin . In: Journal of Hepatology . tape 15 , no. 1-2 , May 1992, pp. 154-161 , doi : 10.1016 / 0168-8278 (92) 90029-O .
- ↑ Dicloxacillin. Retrieved June 8, 2019 .
- ↑ External identifiers or database links for dicloxacillin sodium : CAS number: 343-55-5, EC number: 206-444-3, ECHA InfoCard: 100.005.859 , PubChem : 23667628 , ChemSpider : 58237 , DrugBank : DB00485 , Wikidata : Q27116225 .
- ↑ External identifiers or database links for dicloxacillin sodium hydrate : CAS number: 13412-64-1, EC number: 603-794-2, ECHA InfoCard: 100.111.945 , PubChem : 23675786 , ChemSpider : 24187 , Wikidata : Q27887689 . ( Dicloxacillin Sodium Ph.Eur. )
- ↑ Jorge Casado, Kevin Brigden, David Santillo, Paul Johnston: Screening of pesticides and veterinary drugs in small streams in the European Union by liquid chromatography high resolution mass spectrometry . In: Science of The Total Environment . tape 670 , June 2019, p. 1204–1225 , doi : 10.1016 / j.scitotenv.2019.03.207 .