Kanamycins
The kanamycins are a group of structurally closely related glycosides . In all cases, the 2-deoxystreptamine (middle part of the structure) is glycosidically linked to a 3- D -glucosamine derivative via the 4-OH group .
Commercially available kanamycin mainly containing kanamycin A, but is a mixture of the kanamycins A, B and C. There is a prescription aminoglycoside - antibiotic from Streptomyces ( Streptomyces kanamyceticus ). Kanamycin was first obtained from it in 1957. The first commercial preparations were kanamycin (from Grunenthal ), Kanamytrex (from Boehringer Ingelheim ) and Resistomycin (from Bayer ).
Tobramycin is structurally closely related to the kanamycins. It can be synthesized from Kanamycin B or alternatively obtained from fermentation solutions of Streptomyces tenebarius . Tobramycin is a well-suited aminoglycoside antibiotic for treating Pseudomonas aeruginosa infections. Tobramycin is used both as an intravenous short infusion (30-60min) and in the form of inhalation therapy in cystic fibrosis patients with a Pseudomonas aeruginosa colonization.
properties
Kanamycins are basic, strongly polar oligosaccharides. They are colorless, readily soluble in water and stable in solution in the pH range 2.2–10.0.
| Kanamycins | ||||||||||
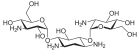
| Surname | Kanamycin A | Kanamycin B | Kanamycin C | Tobramycin | Dibekacin | |||||
| Structural formula |

|

|
|

|
|
|||||
| CAS number | 8063-07-8 64013-70-3 (disulfate) |
4696-76-8 | 2280-32-2 | 32986-56-4 | 34493-98-6 | |||||
| PubChem | 6032 | 439318 | 439582 | 36294 | 470999 | |||||
| Molecular formula | C 18 H 36 N 4 O 11 | C 18 H 37 N 5 O 10 | C 18 H 36 N 4 O 11 | C 18 H 37 N 5 O 9 | C 18 H 37 N 5 O 8 | |||||
| Molar mass | 484.50 g mol −1 | 483.52 g mol −1 | 484.50 g mol −1 | 467.52 g mol −1 | 451.52 g mol −1 | |||||
| Physical state | firmly | |||||||||
|
GHS labeling |
Disulfate
|
sulfate
|
|
|
|
|||||
| H and P phrases | 360 | no H-phrases | see above | no H-phrases | see above | |||||
| no EUH phrases | no EUH phrases | see above | no EUH phrases | see above | ||||||
| 201-308 + 313 | see above | see above | no P-phrases | see above | ||||||
| Toxicological data | > 4000 mg kg −1 ( LD 50 , rat , oral ) | > 7500 mg kg −1 ( LD 50 , rat , oral ) | ||||||||
use
In Germany, Kanamycin is used in human medicine as a sulfate salt in the form of eye drops and ointments for the local treatment of bacterial infections of the eye (e.g. conjunctivitis ). In the US, kanamycin and dosage forms for oral and parenteral application (trade name Kantrex ) in trade.
In veterinary medicine, kanamycin is used as a reserve antibiotic for the treatment of gastrointestinal infections caused by kanamycin-sensitive pathogens in dogs and cats and in combination with spiramycin in acute and chronic mastitis resistant to other therapies . In human medicine, kanamycin is used as a reserve antibiotic, among other things, for the treatment of multi-resistant tuberculosis .
It is also widely used in molecular biology as a selection antibiotic . Genetically modified microorganisms, primarily Escherichia coli, are equipped with kanamycin resistance genes in addition to the genes of interest. Thus, a selection of modified versus native microorganisms is allowed by culturing in media containing kanamycin. In order to cultivate such transgenic E. coli bacteria selectively, for example in LB medium , a final concentration of about 50 μg / ml medium is used. Other antibiotics such as ampicillin or tetracyclines are also used for selection media.
Unlike most other antibiotics, kanamycin is also toxic to plants. Accordingly, when working with transgenic plants and carrying out a corresponding test, kanamycin selection media can also be used.
Mode of action
Kanamycin penetrates bacterial cell membranes by passive diffusion or by (oxygen-dependent) active transport. It attaches itself to the 30S subunit of membrane-associated ribosomes and thus inhibits bacterial protein synthesis .
Analytics
Coupling of HPLC with mass spectrometry is suitable for reliable determination after adequate sample preparation . Also immunoassays can be used.
Trade names
- Kanamycin monopreparations
Kanamycin is available in Germany under the following names:
- for topical application (eye ointment / eye drops):
- Kanamycin POS
- Kanamyxin (Grünenthal)
- Tobramycin monopreparations
Tobramycin is available in Germany under the following names:
- for inhalation in cystic fibrosis :
- Tobi
- Bramitob
- Gernebcin
- for topical application (ointment):
- Tobramaxine
- for parenteral use
- Tobramycin B. Braun
- Gernebcin
- Tobrazide
Individual evidence
- ↑ Karl Wurm, AM Walter: Infectious Diseases. In: Ludwig Heilmeyer (ed.): Textbook of internal medicine. Springer-Verlag, Berlin / Göttingen / Heidelberg 1955; 2nd edition, ibid. 1961, pp. 9–223, here: p. 54.
- ↑ Karl Wurm, AM Walter: Infectious Diseases. In: Ludwig Heilmeyer (ed.): Textbook of internal medicine. 1961, p. 54.
- ^ Theodor Dingermann, Rudolf Hänsel, Ilse Zündorf (eds.): Pharmaceutical Biology: Molecular Basics and Clinical Applications. 1st edition. Springer Verlag, Berlin 2002, ISBN 3-540-42844-5 , p. 309 ff.
- ↑ Data sheet Kanamycin disulfate salt from Streptomyces kanamyceticus from Sigma-Aldrich , accessed on May 3, 2011 ( PDF ).
- ↑ Data sheet Kanamycin B sulfate salt from Sigma-Aldrich , accessed on May 3, 2011 ( PDF ).
- ↑ Data tobramycin at Sigma-Aldrich , accessed 3 May 2011 ( PDF ).
- ↑ Entry on Kanamycine in the DrugBank of the University of Alberta .
- ↑ Data sheet Tobramycin, Free Base (PDF) from Calbiochem, accessed on December 8, 2015.
- ↑ JA Dijkstra, MG Sturkenboom, K. v. Hateren, RA Koster, B. Greijdanus, JW Alffenaar: Quantification of amikacin and kanamycin in serum using a simple and validated LC-MS / MS method. In: Bioanalysis. 6 (16). Aug 2014, pp. 2125-2133. PMID 25331857
- ↑ Q. Gong, L. Ding, S. Zhu, Y. Jiao, J. Cheng, S. Fu, L. Wang: Determination of ten aminoglycoside residues in milk and dairy products using high performance liquid chromatography-tandem mass spectrometry. In: Se Pu. 30 (11), Nov 2012, p. 11437. PMID 23451516 (Chinese)
- ↑ P. Kumar, A. Rúbies, R. Companyó, F. Centrich: Determination of aminoglycoside residues in kidney and honey samples by hydrophilic interaction chromatography-tandem mass spectrometry. In: J Sep Sci. 35 (20), Oct 2012, pp. 2710-2717. PMID 23065931
- ↑ JA Dijkstra, AJ Voerman, B. Greijdanus, DJ Touw, JW Alffenaar: Immunoassay Analysis of Kanamycin in Serum Using the Tobramycin Kit. In: Antimicrob Agents Chemother. 60 (8), Jul 22, 2016, pp. 4646-4651. PMID 27185806
