Spiramycin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
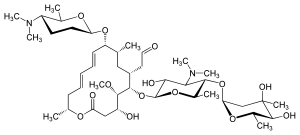
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Spiramycin | |||||||||||||||
| other names |
[(4 R , 5 S , 6 S , 7 R , 9 R , 10 R , 11 E , 13 E , 16 R ) -6 - {[(2 S , 3 R , 4 R , 5 S , 6 R ) -5 - {[(2 S , 4 R , 5 S , 6 S ) -4,5-dihydroxy-4,6-dimethyl- tetrahydro-2 H -pyran-2-yl] oxy} -4- (dimethylamino) -3 -hydroxy-6-methyl-tetrahydro-2 H -pyran-2-yl] oxy} -10 - {[(2 S , 5 S , 6 R ) -5- (dimethylamino) -6-methyl-tetrahydro-2H -pyran-2-yl] oxy} -4-hydroxy-5-methoxy-9,16-dimethyl-2-oxooxacyclohexadeca-11,13-dien-7-yl] acetaldehyde ( IUPAC for spiramycin I) |
|||||||||||||||
| Molecular formula | C 43 H 74 N 2 O 14 | |||||||||||||||
| Brief description |
white to slightly yellowish powder, slightly hygroscopic |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class |
Macrolide antibiotic |
|||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 843.05 g · mol -1 | |||||||||||||||
| solubility |
slightly soluble in water, slightly soluble in acetone , ethanol and methanol |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Spiramycin is a macrolide antibiotic . It was first obtained from the Streptomyces species Streptomyces ambofaciens found in northern France and traded as Selectomycin by Grünenthal . works against various types of bacteria and can also be used for toxoplasmosis . Nowadays, toxoplasmosis in pregnancy is usually the only indication for spiramycin.
effect
Spiramycin binds to the 50S subunit of prokaryotic ribosomes . The mechanism of observed resistances is not yet fully understood and can vary depending on the organism.
properties
Pharmaceutical grades of spiramycin are obtained from certain strains of Streptomyces ambofaciens or produced by other processes.
The main component is spiramycin I; Spiramycin II (4- O- acetyl-spiramycin I) and spiramycin III (4- O- propanoyl-spiramycin I) are also present.
- Composition (as a dried substance)
- Spiramycin I: at least 80.0%
- Spiramycin II: not more than 5.0%
- Spiramycin III: maximum 10.0%
- Sum of the contents of spiramycin I, II and III: at least 90.0%
Various structurally related impurities such as spiramycin IV, spiramycin V, spiramycin dimer , neospiramycin I, neospiramicin II and neospiramicin III (each maximum 2.0%, in total maximum 10.0%).
Trade names
Human medicine
- Monopreparations : Rovamycine (A, D, CH), Selectomycin (D)
Veterinary medicine
- Monopreparations : Spiramastine ad us. vet. (CH), Suanovil 20 ad us. vet. (CH)
- Combination preparations : Suanatem ad us. vet. (Dogs) VET with metronidazole (D), Kanamastine 400 ad us. vet. with Kanamycin (CH), Stomorgyl ad us. vet. with metronidazole (CH)
Web links
- Entries in the NIH study register
- Taking spiramycin during pregnancy and breastfeeding
- Spiramycin - information from the Robert Koch Institute
- Entry on spiramycin at Vetpharm, accessed July 2, 2010.
Individual evidence
- ↑ a b c data sheet SPIRAMYCIN CRS (PDF) at EDQM , accessed on July 2, 2010.
- ↑ Spiramycin data sheet from Sigma-Aldrich , accessed on April 23, 2011 ( PDF ).
- ↑ Karl Wurm, AM Walter: Infectious Diseases. In: Ludwig Heilmeyer (ed.): Textbook of internal medicine. Springer-Verlag, Berlin / Göttingen / Heidelberg 1955; 2nd edition, ibid. 1961, pp. 9–223, here: p. 53.
- ↑ Vassol St Georgiev: Opportunistic Infections (Infectious Disease (Totowa, NJ).) . Humana Press, 2001, ISBN 1-58829-009-3 ( limited preview in Google Book Search).
- ↑ JL Pernodet, MT Alegre, MH Blondelet-Rouault, M. Guérineau: Resistance to spiramycin in Streptomyces ambofaciens, the producer organism, involves at least two different mechanisms . In: Journal of General Microbiology . tape 139 , no. 5 , May 1993, pp. 1003-1011 , doi : 10.1099 / 00221287-139-5-1003 , PMID 7687646 .
- ↑ Y. Nakajima: Mechanisms of bacterial resistance to macrolide antibiotics . In: Journal of Infection and Chemotherapy . tape 5 , no. 2 , June 1999, p. 61-74 , doi : 10.1007 / s101560050011 , PMID 11810493 .
- ↑ a b c EDQM (Ed.): European Pharmacopoeia 9.0 (2016) . Spiramycin (Monograph 01/2017: 0293). S. 3635 ff .