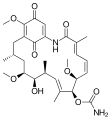
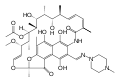
Ansamycine
Ansamycins are macrocyclic , antibiotically effective natural products that are structurally characterized by a aromatic component in which non-adjacent positions by an aliphatic bridged chain to form a cyclic structure (ansa compound Phan ). One of the aliphatic-aromatic bridging points is always an amide bond .
The name goes back to Vladimir Prelog and Wolfgang Oppolzer and is derived from the Latin "ansa" ( handle , handle).
Occurrence
Ansamycins are found in Streptomyces , Nocardia and Micromonospora and have also been isolated from plant material.
Classification
The naturally occurring ansamycins can be divided depending on the type of the aromatic component ( benzene derivative / benzoquinone , Naphthalinabkömmling / naphthoquinone ) and the chain length of the aliphatic component. Around 120 representatives were identified and characterized (as of 2000). The main group is made up of the representatives of the naphthalene C 17 group.
| Aromatic component | Benzene | Naphthalene | |||
| Length of the "Ansa" chain | C 15 | C 17 | C 17 | C 23 | C 9 |
|
Biogenic ansamycins |
Ansatrienin |
Ansathiazin |
Actamycin |
||
In addition to the naturally occurring ansamycins, variants have also been isolated from mutant strains or produced semisynthetically .
Selected representatives:
meaning
Representatives of the naphthalene-C 17 group show a selective effect against bacteria by inhibiting their RNA polymerase . The rifamycins are therapeutically important. Representatives of the benzene-C 15 group such as the ansamitocine and maytansine have an anti- tumor effect . The cytostatic maytansine derivative DM1 ( mertansine ) is used as an antibody-drug conjugate with trastuzumab (T-DM1, trastuzumab-emtansine) for the treatment of HER2 / neu -positive breast cancer .
literature
- Descripions on main antibiotic types . In: Barrie W. Bycroft (Ed.): Dictionary of Antibiotics & Related Substances . Chapman & Hall, London, New York, 1988. S. xiv. ( limited preview in Google Book search)
- S. Funayama, GA Cordell: Ansamycin Antibiotics Discovery, Classification, Biosynthesis and Biological Activities . In: Atta-ur-Rahman (Ed.): Studies in Natual Products Chemistry. Volume 23: Bioactive Natural Products (Part D) . Elsevier, Amsterdam, 2000. ( limited preview in Google book search)
- A. Stratmann: The biology of the ansamycins: example rifamycin. Anbics Laboratories AG, Martinsried. In: BIOspektrum, Volume 10 (2004), Edition 3, p. 249 ( PDF )
Individual evidence
- ↑ I. Krop, EP Winer: Trastuzumab Emtansine: A Novel Antibody-Drug Conjugate for HER2-Positive Breast Cancer. In: Clinical Cancer Research. 20, 2014, p. 15, doi : 10.1158 / 1078-0432.CCR-13-0541 .