Bedaquiline
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
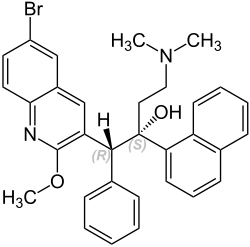
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Bedaquiline | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 32 H 31 BrN 2 O 2 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class |
Tuberculostatics |
|||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 555.51 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Bedaquiline is a drug used to treat tuberculosis ( tuberculostatic ). It is used in particular in combination therapies against multi-resistant tuberculosis bacteria .
Chemically, it is a compound from the group of diarylquinolines .
history
Bedaquiline was approved in the USA at the end of 2012 and in the EU in 2014 , making it the first new tuberculostatic drug in decades. Rifabutin was last added in Germany in 1994.
Bedaquiline emerged from a screening program with more than 70,000 compounds. In 2015, the researchers Koen Andries and Jérôme Guillemont received the European Inventor Award for this .
properties
Bedaquiline has two stereogenic centers . Investigations of the minimum inhibitory concentrations (MIC) of the various isomers showed that the (1 R , 2 S ) stereoisomer (= bedaquiline) was the most effective (its MIC against Mycobacterium tuberculosis was between 0.03 and 0.12 µg / ml; the MIC 90 was 0.06 µg / ml. In comparison, the MIC 90 for the (1 S , 2 R ) isomer was 8.8 µg / ml, that of the mixture of both 1.8 µg / ml and that of the (1 R , 2 R / 1 S , 2 S ) -diastereomer mixture 44 µg / ml). This in-vitro finding is the same as that from computer-aided attachment models. Enantiomerically pure bedaquiline is obtained by controlled crystallization using an enantiomeric organophosphate as the conclusion of the multi-stage synthesis, in which a mixture of the stereoisomers is first formed.
The salt (1: 1) of fumaric acid , bedaquiline fumarate , is used medicinally . The substance is a white to almost white, non-hygroscopic powder that is practically insoluble in aqueous media over a wide pH range , but soluble in a number of organic solvents.
Mechanism of action
Bedaquiline inhibits the ATP synthase of mycobacteria by binding to the c-subunit of the enzyme. The ATP synthase is an important enzyme in the cellular energy metabolism of the mycobacteria. Their inhibition leads to bactericidal effects in both dividing and non-dividing pathogens. Bedaquiline is the only therapeutically used antibiotic with this mechanism of action. The minimum inhibitory concentration ( MIC ) for M. tuberculosis are 0.03 μg / mL (for the MIC 50 ) and 0.06 μg / mL (for the MIC 90 ).
Pharmacokinetics
The main route of degradation is N - demethylation by CYP3A4 . It is assumed that the resulting metabolite (M2) does not contribute significantly to the effectiveness. Bedaquiline is excreted in the faeces .
Side effects
Bedaquiline is both hepato- and cardiotoxic in that it increases liver enzymes ( AST , ALT ) and bilirubin , and increases the QT interval in the heart . The most common side effects observed in the studies were nausea , arthralgia , headache, vomiting, and dizziness.
Finished medicinal products
Sirturo (manufacturer: Janssen Pharmaceutica )
See also
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c Ashish Kumar Kakkar, Neha Dahiya: Bedaquiline for the treatment of resistant tuberculosis: Promises and pitfalls . In: Tuberculosis . tape 94 , no. 4 , 2014, p. 357-362 , doi : 10.1016 / j.tube.2014.04.001 .
- ↑ a b Mageshwaran Lakshmanan, Alphienes Stanley Xavier: Bedaquiline - The first ATP synthase inhibitor against multidrug resistant tuberculosis . In: Journal of Young Pharmacists . tape 5 , no. 4 , 2013, p. 112–115 , doi : 10.1016 / j.jyp.2013.12.002 .
- ↑ New drugs for tuberculosis , November 20, 2016.
- ↑ a b Entry on Bedaquiline. In: Römpp Online . Georg Thieme Verlag, accessed on November 25, 2016.
- ↑ K. Andries et al .: A Diarylquinoline Drug Active on the ATP Synthase of Mycobacterium tuberculosis . In: Science . tape 307 (2005) , pp. 223-227 , doi : 10.1126 / science.1106753 .
- ↑ Koul, A., Dendouga, N., Vergauwen, K. et al .: Diarylquinolines target subunit c of mycobacterial ATP synthase . In: Nature Chemical Biology . tape 3 (2007) , pp. 323-324 , doi : 10.1038 / nchembio884 .
- ↑ Marc R. de Jonge, Luc HM Koymans, Jérôme EG Guillemont, Anil Koul, Koen Andries: A computational model of the inhibition of Mycobacterium tuberculosis ATPase by a new drug candidate R207910 . In: Proteins Structure Function and Bioinformatics . May 1, 2007, doi : 10.1002 / prot.21376 .
- ↑ a b c d CHMP assessment report - Sirturo, dated December 13, 2013.
- ↑ External identifiers from or database links to Bedaquilinfumarat : CAS Number: 845533-86-0, EC number: 805-637-5, ECHA -InfoCard: 100232531 , PubChem : 24812732 , ChemSpider : 28528191 , Wikidata : Q27139886 .
- Jump up ↑ Ke Liu, Feng Li, Jie Lu, Shinlan Liu, Kenneth Dorko, Wen Xie, Xiaochao Ma: Bedaquiline Metabolism: Enzymes and Novel Metabolites . In: Drug Metabolism and Disposition . tape 42 , no. 5 , May 1, 2014, p. 863-866 , doi : 10.1124 / dmd.113.056119 .