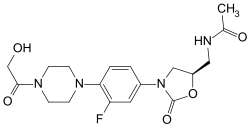
Eperezolid
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Non-proprietary name | Eperezolid | |||||||||
| other names |
( S ) - N - [(3- (3-Fluoro-4 (4- (hydroxyacetyl) -1-piperazinyl) phenyl) -2-oxo-5-oxazolidinyl) methyl] acetamide |
|||||||||
| Molecular formula | C 18 H 23 FN 4 O 5 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| Drug information | ||||||||||
| Drug class |
antibiotic |
|||||||||
| properties | ||||||||||
| Molar mass | 394.40 g · mol -1 | |||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Eperezolid is an antibiotic from the group of oxazolidinones . It inhibits protein synthesis in gram-positive bacteria. It is particularly effective against methicillin-resistant Staphylococcus aureus , enterococci and streptococci .
pharmacology
Eperezolid inhibits protein synthesis at a very early point in the initiation step. Various proteins ( initiation factors ) combine with the t-RNA to form an initiation complex. Eperezolid interferes with the precise formation of this complex; as a result, the proteins are not expressed. The gram-negative bacterial cell wall is less permeable to eperezolid than the gram-positive.
literature
- Synthesis: Schaus, SE. and Jacobsen, EN. (1996): Dynamic Kinetic Resolution of Epichlorohydrin via Enantioselective Catalytic Ring Opening with TMSN3. Practical Synthesis of Aryl Oxazolidinone Antibacterial Agents . In: Tetrahedron Letters 37 (44), 7937-7940; doi : 10.1016 / 0040-4039 (96) 01835-7
- Pharmacology:
- Hutchinson, DK. (2003): Oxazolidinone Antibacterial Agents: A Critical Review . In: Curr Top Med Chem . 3 (9): 1021-42; PMID 12678835
- Rybak, MJ. et al. (1998): Comparative in vitro activities and postantibiotic effects of the oxazolidinone compounds eperezolid (PNU-100592) and linezolid (PNU-100766) versus vancomycin against Staphylococcus aureus, coagulase-negative staphylococci, Enterococcus faecalis, and Enterococcus faecium. (PDF; 217 kB) In: Antimicrob Agents Chemother . 42 (3); 721-4; PMID 9517963
- Usage: Tucker, YES. et al. (1998): Piperazinyl oxazolidinone antibacterial agents containing a pyridine, diazene, or triazene heteroaromatic ring . In: J Med Chem. 41 (19); 3727-35; PMID 9733498
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.