Azlocillin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
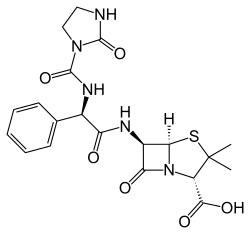
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Azlocillin | |||||||||||||||||||||
| other names |
(2 S , 5 R , 6 R ) -3,3-Dimethyl-7-oxo-6 - {[(2R) -2 - {[(2-oxo-1-imidazolidinyl) carbonyl] amino} -2-phenylacetyl ] amino} -4-thia-1-azabicyclo [3.2.0] heptane-2-carboxylic acid ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 20 H 23 N 5 O 6 S | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 461.49 g mol −1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Azlocillin is an antibiotic from the group of Β-lactam antibiotics . It belongs to the penicillins, specifically to the subgroup of ureidopenicillins (acylaminopenicillins).
Chemically, azlocillin is derived from the aminopenicillin ampicillin , from which it differs by attaching a ureido pyrazolidinone group. Like all ureidopenicillins, azlocillin is highly effective against Pseudomonas aeruginosa , but is not beta-lactamase- resistant.
literature
- Hager's Handbook of Pharmaceutical Practice : Substances A – D; Springer, ISBN 978-3-642-63429-1 , page limited preview in the Google book search
Individual evidence
- ↑ a b azlocillin. In: Sigma-Aldrich. Accessed March 27, 2020 (English).
- ↑ H. Lode, U. Niestrath, P. Koeppe, H. Langmaack: [Azlocillin and mezlocillin: two new semisynthetic acylureido-penicillins (author's transl)] . In: Infection . tape 5 , no. 3 , 1977, ISSN 0300-8126 , p. 163-169 , doi : 10.1007 / bf01639753 , PMID 334673 .
- ^ R. Wise, AP Gillett, JM Andrews, KA Bedford: Activity of Azlocillin and Mezlocillin Against Gram-Negative Organisms: Comparison with Other Penicillins . In: Antimicrobial Agents and Chemotherapy . tape 13 , no. 4 , April 1978, ISSN 0066-4804 , pp. 559-565 , PMID 96726 , PMC 352288 (free full text).