Piperacillin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
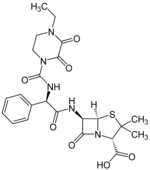
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Piperacillin | |||||||||||||||||||||
| other names |
(2 S , 5 R , 6 R ) -6 - [( R ) -2- (4-Ethyl-2,3-dioxopiperazine-1-carboxamido) -2-phenylacetamido] -3,3-dimethyl-7-oxo -4-thia-1-azabicyclo [3.2.0] heptane-2-carboxylic acid ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 23 H 27 N 5 O 7 S | |||||||||||||||||||||
| Brief description |
White to almost white powder |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action |
Inhibition of septum formation and cell wall synthesis |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 517.56 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
183–185 ° C (decomposition) (piperacillin sodium salt) |
|||||||||||||||||||||
| solubility |
Slightly soluble in water , slightly soluble in methanol , sparingly soluble in ethyl acetate |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Piperacillin is a β-lactam antibiotic which has a four-membered lactam ring in its structural formula and belongs to the group of acylaminopenicillins . This active ingredient has the broadest spectrum of activity of all penicillins (including Pseudomonas and Enterobacteria ).
Clinical information
Spectrum of activity
The spectrum of activity of piperacillin goes beyond that of benzylpenicillin . It works against gram-negative rods , enterobacteria and anaerobes . The effectiveness against gram-positive cocci is worse than that of benzylpenicillin, but is considered sufficient and is comparable to that of amoxicillin .
Application areas (indications)
For the treatment of acute and chronic bacterial infections of various localization and intensity caused by pathogens sensitive to piperacillin, such as:
- Treatment of infections in which gram-negative rods are suspected, such as urogenital infections, including pyelonephritis , cystitis and urethritis . Piperacillin is also effective for acute, uncomplicated infections caused by Neisseria gonorrhoeae , including prostatitis
- intra-abdominal infections, such as biliary tract infections , peritonitis and intra-abdominal abscesses (often caused by gram-negative and / or anaerobic organisms of the normal intestinal flora )
- gynecological infections such as endometritis , abscesses and inflammations of the pelvis, adnexitis
- Pneumonia ventilated patients or with pneumonia with Pseudomonas aeruginosa as probable pathogens
- severe sepsis , bacterial endocarditis
- Skin and soft tissue infections, including post-accident, surgical, and infected burn infections
- Bone and joint infections - including osteomyelitis
- Septicemia (term for the total infection of the human organism caused by bacteria or toxins in the blood)
- nosocomial infections caused by the pathogen Pseudomonas aeruginosa , here piperacillin is the preferred penicillin for calculated chemotherapy (initial therapy if a germ analysis is not yet available, which is based on the expected germ spectrum)
Contraindications (contraindications)
Because of the risk of anaphylactic shock , piperacillin must not be used in patients with proven penicillin hypersensitivity. A cross-allergy with other β-lactam antibiotics may exist. In patients with allergic diseases in history as bronchial asthma , allergic rhinitis , urticaria (hives), the risk of severe hypersensitivity reactions at injection - or infusion treatment increases, so Piperacillin should be applied in such cases if clearly indicated with caution.
- Use during pregnancy and breastfeeding:
As there is no experience of its use in humans during pregnancy and breastfeeding, piperacillin should not be used during pregnancy and breastfeeding .
Adverse effects (side effects)
The following are the most important things about possible, known side effects of piperacillin. These adverse drug reactions do not have to occur, but they can. Every patient reacts differently to medication.
- Uncommon side effects:
Drug eruptions as external phenomena ( manifestations ) of a drug allergy or a pseudoallergy in the form of rashes, reddening of the skin, itching and inflammation of the mucous membranes ; Purpura (small speckled capillary bleeding into the skin , subcutis or mucous membranes , which occurs with high doses); Serum creatinine surge; Increase in blood urea concentration; A headache.
- Rare side effects:
Severe allergic reactions such as drug fever ; Joint pain; Eosinophilia ; angioneurotic edema ( Quincke's edema ); Swelling of the throat; Serum sickness ; Anemia due to the breakdown of red blood cells; allergic vascular inflammation; Inflammation of the kidneys; Erythema exudativum multiforme ; Stevens-Johnson Syndrome ; decreased hemoglobin ( anemia ); Lack of solid blood components; essential thrombocythemia d. H. an excessive increase in the number of thrombocytes (platelets) in the blood.
Pharmacological properties
Pharmacokinetics
The acylaminopenicillins are not β-lactamase - and acid-stable. They are not absorbed after oral administration and must be administered parenterally - either intravenously or intramuscularly . After an intravenous bolus injection of 1 gram of piperacillin, plasma concentrations of 70 mg · L −1 are measured, with higher doses concentrations of 400 mg · L −1 or more can be achieved. Due to the rapid penetration into the bacterial cell wall , good crypticity is achieved. The maximum plasma levels are lower after a short intravenous infusion. The plasma protein binding is 20% and the bioavailability is 100% with intravenous, 70 to 80% with intramuscular administration. Since the active ingredient is rapidly and largely unchanged with an elimination half-life of 65 minutes, 80% (by glomerular filtration and tubular secretion) is eliminated renally , and to a proportion of 6 to 15% by biliary excretion , the concentrations decrease rapidly.
By combining the β-lactamase-labile piperacillin with a β-lactamase inhibitor such as tazobactam , the antibiotic resistance of bacteria against β-lactamase-labile penicillins can be overcome (there is also an expanded spectrum for the initial therapy of life-threatening abdominal infections ). β-lactamase inhibitors have little or no direct antibacterial activity. They irreversibly block certain clinically common β-lactamases. Most plasmid-mediated penicillinases are inhibited, less or not at all the chromosomally mediated cephalosporinases. The pharmacokinetic properties of the two combination partners are similar (the combination with an aminoglycoside or a second-generation quinolone is recommended for the treatment of Pseudomonas infections ).
Trade names
numerous generics (D)
Piperacillin / tazobactam in the ratio 8: 1 (D, A, CH), Pipitaz (A), Tazobac (D, CH), Tazonam (A)
literature
- Ernst Mutschler , Gerd Geisslinger, Heyo K. Kroemer , Sabine Menzel, Peter Ruth: Mutschler drug effects. Pharmacology - Clinical Pharmacology - Toxicology , 10th Edition. Wissenschaftliche Verlagsgesellschaft, Stuttgart 2012, ISBN 3-80-472898-7 .
Web links
- Piperacillin - a new broad-spectrum penicillin article in the journal of chemotherapy
Individual evidence
- ↑ a b European Pharmacopoeia Commission (ed.): EUROPEAN PHARMACOPOE 5TH EDITION . tape 5.0 - 5.7 , 2006.
- ^ The Merck Index. An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, p. 1286, ISBN 978-0-911910-00-1 .
- ↑ a b c d data sheet Piperacillin sodium salt from Sigma-Aldrich , accessed on June 16, 2011 ( PDF ).
- ↑ a b c d Entry on piperacillin. In: Römpp Online . Georg Thieme Verlag, accessed on May 1, 2014.
- ↑ Information for professionals on Piperacillin Fresenius .
- ↑ Jörg Braun: Infectious Diseases. In: Jörg Braun, Roland Preuss (Ed.): Clinic Guide Intensive Care Medicine. 9th edition. Elsevier, Munich 2016, ISBN 978-3-437-23763-8 , pp. 437-519, here: pp. 485 f. ( Piperacillin / tazobactam ).
- ↑ Jörg Braun: Infectious Diseases. 2016, p. 486.
- ↑ Holmes, B. et al. (1984): Piperacillin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. Vol. 28, pp. 375-425, PMID 6391888 .