Butane
The butanes form a group of substances within the alkanes , which have the empirical formula C 4 H 10 . It consists of the two representatives n- butane and iso- butane , which are isomeric to one another . Both butanes are colorless, flammable, easily liquefied gases (" liquefied gases ") that hardly dissolve in water, but dissolve well in ethanol and ether .
| Properties of butanes | ||
| Surname | n -Butane | Isobutane |
| Refrigerant | R-600 | R-600a |
| Structural formula |
|
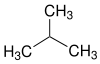
|
| CAS number | 106-97-8 | 75-28-5 |
| ECHA InfoCard | 100.003.136 | 100,000,780 |
| PubChem | 7843 | 6360 |
| Molecular formula | C 4 H 10 | |
| Molar mass | 58.12 g mol −1 | |
| Melting point | −138.29 ° C | −159.42 ° C |
| boiling point | −0.50 ° C | −11.7 ° C |
| Vapor pressure (20 ° C) | 2081 hPa | 3019 hPa |
| Density (gas, 0 ° C, 1013 mbar) | 2.71 kg m −3 | 2.70 kg m −3 |
| Density (liquid at boiling point) | 0.59 kg l −1 | 0.60 kg l −1 |
| calorific value | 46 MJ kg −1 (12.72 kWh kg −1 ) 123 MJ m −3 (34.32 kWh m −3 ) |
|
| Solubility in water at 20 ° C | 61 mg l −1 | 49 mg l −1 |
|
Lower explosion limit (LEL) |
1.4% by volume | 1.5% by volume |
| 33 g m −3 | 37 g m −3 | |
|
Upper explosion limit (UEL) |
9.4% by volume | |
| 231 g m −3 | ||
Occurrence and representation
Butanes occur naturally in natural gas , but are also obtained from crude oil by cracking . The two isomers can be separated by adsorption and fractional desorption from activated carbon or zeolites . Isobutane is produced in large quantities from n -butane by isomerization with a mixture of aluminum chloride and hydrogen chloride as a catalyst .
use
Butane are used as fuel gas , (e.g., lighter gas.) Refrigerant (isobutane: R600a) as propellants , food additive and extraction means (for example CBD used to extract). Gas mixtures, for example of 40% propane and 60% butane, are used at filling stations as autogas , as bottled gas for gas rechauds and for technical devices for soldering and welding , as gas cartridges in camping areas and as refill cans for lighters and the like.
In winter gasoline fuel (is gasoline ) with iso - butane and additional sold, as these substances because of the low boiling point of the Kaltstartfreudigkeit the gasoline engine to improve at low outside temperatures (see also motor gasoline # production ).
Butane be in the chemical industry for the preparation of C 4 alkenes such as 1,3-butadiene , 1-butene , 2-butene and isobutene and higher for syntheses hydrocarbons (for " alkylate ") thiophene as well as oxidation products such as tert -butyl hydroperoxide employed .
safety instructions
Like all alkanes , butanes have a narcotic and oxygen-displacing effect. In the case of respiratory depression triggered by this , effects on the central nervous system such as excitement, euphoria and vomiting can occur, with high doses also negative effects on the blood circulation and heart (such as cardiac arrhythmias ).
Individual evidence
- ↑ a b c Wissenschaft-Online-Lexika: Entry on "Butane" in the Lexikon der Chemie, accessed on November 17, 2011.
- ^ Entry on butane in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ Entry on isobutane in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ Refrigerant gefahrgutbrumme.de, accessed December 23, 2019.
- ↑ a b Entry on butanes. In: Römpp Online . Georg Thieme Verlag, accessed on November 17, 2011.
- ↑ a b food dictionary
