Phenolphthalein
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Phenolphthalein | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 20 H 14 O 4 | |||||||||||||||||||||
| Brief description |
white to pale yellow, crystalline, tasteless and odorless solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 318.31 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.30 g cm −3 |
|||||||||||||||||||||
| Melting point |
263.7 ° C |
|||||||||||||||||||||
| pK s value |
9.7 |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Authorization procedure under REACH |
particularly worrying : carcinogenic ( CMR ) |
|||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Phenolphthalein is one of the best-known pH indicators and was first presented in 1871 by Adolf von Baeyer . The name is made up of phenol and phthalic anhydride . Phenolphthalein is a triphenylmethine dye (outdated term triphenylmethane dye ) and forms the basic compound of the phthaleine family. The corresponding sulfonphthalein is phenol red . At a pH of less than 0, an aqueous solution of phenolphthalein is red-orange, at a pH value of 0 to about 8.2 colorless. At a higher pH value, the solution turns pink-violet, whereby it becomes colorless again at a pH value of about 13.
presentation
In a Friedel-Crafts acylation , two equivalents of phenol and one equivalent of phthalic anhydride are reacted in the presence of small amounts of concentrated sulfuric acid or zinc chloride:
properties
Phenolphthalein is a white crystalline powder and is practically insoluble in water. It is mostly used in a 1% alcoholic solution. It is a weak acid itself.
Phenolphthalein has a pK s value of 9.7. If the transition range is determined with an indicator acid / base ratio of 1:10 to 10: 1, according to the Henderson-Hasselbalch equation, a transition range of pH = pK s ± 1 (8.7 to 10.7) is obtained. At a pH value of less than 0 an aqueous phenolphthalein solution is red-orange, at a pH value of 0 to about 8.2 it is colorless, at a higher pH value the solution turns pink-violet in the strongly alkaline range - at a pH value close to 13 - it becomes colorless again. It is therefore well suited as an indicator for the titration of basic solutions, for example .
Structure and color change
Depending on the pH of the solution, the phenolphthalein changes its structure and thus its color.
| species | H 3 In + | H 2 In | In 2− | In (OH) 3− |
|---|---|---|---|---|
| structure | 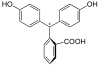 |
 |
 |

|
| pH | <0 | 0 to 8.2 | 8.2 to 12.0 | > 12.0 |
| colour | red | colorless | pink-purple | colorless |
 |
 |
In the pH range up to about 7.5 it is in its colorless, uncharged basic form (H 2 In 1 ). In a more basic solution, the protons are split off from the two hydroxyl groups. A quinoid system is present as a chromophore in a resulting mesomeric boundary structure (In 2− 2 ). This is the colored structure of the indicator. In strongly basic environment is an OH group thereby achieving the chromophore becomes impossible (In (OH) anneals to the central carbon atom, 3- 3 ). Phenolphthalein turns color again in a strongly acidic solution. The lactone ring is cleaved by the H + . A positive charge is formed on the central carbon atom, which is thus sp 2 -hybridized and is thus stabilized again by the mesomeric limit shape ( 4 ).
If you consider the reaction equation of reaction (A) to (B), the law of mass action makes it clear why the color change occurs so quickly:
The following applies (K s constant, whereby the quasi-constant concentration of the water is included in K s ):
The concentration of the H 3 O + ions is of a different order of magnitude. It is high for acidic solutions, the equilibrium is on the side of (A). But as soon as the concentration of H 3 O + becomes very small or the concentration of OH - becomes large, the concentration of (B) must increase massively because of the constancy of the term. Because (B) arises from (A), the concentration of (A) becomes extremely much smaller - the color change takes place very quickly.
Further use
For the reliable qualitative and quantitative detection of phenolphthalein in forensic issues, the coupling of HPLC with mass spectrometry is used. The detection limit for this method is given as 1.66 pg / L and is also used to identify the substance in allegedly purely herbal slimming products .
Reduced phenolphthalein is used in the Kastle Meyer test to detect traces of blood in forensics .
Phenolphthalein was used as a laxative for more than a hundred years before it was discovered that it was potentially carcinogenic . However, the small amounts used when used as an indicator are not dangerous. In construction, phenolphthalein solution is used to visualize the depth of carbonation on concrete and to determine whether newly plastered surfaces can be recoated (surfaces with a pH value of less than 8.5).
Individual evidence
- ↑ a b Entry on phenolphthalein. In: Römpp Online . Georg Thieme Verlag, accessed on November 10, 2014.
- ↑ a b c d e Entry on phenolphthalein in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ a b Entry on phenolphthalein in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Entry on phenolphthalein in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on July 16, 2014.
- ^ The New Encyclopæedia Britannica; Micropæedia Vol. VII; 15th Ed .; Encyclopedia Britannica, Inc. (1974).
- ^ Phenolphthalein at Seilnacht.com, accessed February 4, 2013.
- ↑ K. Sharma, SP Sharma, SC Lahiri: Detection and quantitation of trace phenolphthalein (in pharmaceutical preparations and in forensic exhibits) by liquid chromatography-tandem mass spectrometry, a sensitive and accurate method. In: J Forensic Sci . 58 Suppl 1, Jan 2013, pp. S208-S214. PMID 23106487 .





