Phenol red
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Phenol red | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 19 H 14 O 5 S | |||||||||||||||
| Brief description |
red solid with a faint odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 354.38 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
> 300 ° C |
|||||||||||||||
| solubility |
very bad in water (0.77 g l −1 at 100 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Phenol red is a triphenylmethane dye and forms the basic compound of the sulfonphthalein family . It is used as a pH indicator and has two transition areas. At pH ≈ 0.9 the color changes from red to yellow and at pH ≈ 6.4–8.2 from yellow to red-violet.
presentation
In a Friedel-Crafts acylation , two equivalents of phenol and one equivalent of 2-sulfobenzoic anhydride are reacted in the presence of small amounts of concentrated sulfuric acid or zinc chloride.
properties
Phenol red is a red solid with a faint odor.
Phenol red contains two hydroxyl groups and an unstable sultone ring . This ring is split in an aqueous medium, and after a rearrangement the quinoid yellow colored form of the dye is formed. This quinoid system can be protonated in a strongly acidic environment (pH <1), causing the solution to turn red. In a neutral environment (pH = 6.4-8.3) the hydroxyl group is deprotonated and the solution turns red-violet. In the strongly basic range (pH> 14) an addition of an OH group takes place with the formation of a triphenylmethanol structure. Phenol red is then present as a colorless trianion.
| species | H 2 In | In - | In 2− |
|---|---|---|---|
| structure |  |
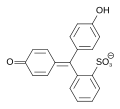 |

|
| pH | <1 | 1-7.3 | > 7.3 |
| colour | red | yellow | Red-violet |
use
Phenol red is used as an indicator in acid-base titrations , with a transition range between pH 6.4 and 8.2. The color changes from red-violet in the basic to yellow in the acidic. Since phenol red dissolves very poorly in water, the ready-to-use solution is prepared either by
- Dissolve 0.1 g in 100 ml of 20 percent ethanol or through
- Dissolve 0.04 g in 1.13 ml sodium hydroxide solution (0.1 mol / l) and then make up to 100 ml with water.
Phenol red serves as a pH indicator for cell culture media .
Individual evidence
- ↑ a b c d e f data sheet phenol red (PDF) from Merck , accessed on January 3, 2013.
- ↑ a b Udo R. Kunze: Fundamentals of quantitative analysis. 3. Edition. Georg Thieme Verlag, Stuttgart 1990, p. 96.
- ↑ Markus Weinmann, Max Planck Institute: Sulfonphtaleine - Phenolrot ( Memento from July 8, 2007 in the Internet Archive )
- ↑ K. Yamaguchi, Z. Tamura, M. Maeda: Molecular Structure of the Zwitterionic Form of Phenolsulfonphthalein. In: Analytical Sciences . 1997, 13 (3). Pp. 521-522. doi: 10.2116 / analsci.13.521
- ↑ BDSoft: Phenol red
- ↑ chemie-master.de: Phenol red
literature
- Z. Tamura, M. Maeda: Differences between phthaleins and sulfonphthaleins. In: Yakugaku Zasshi. 1997, 117 (10-11), pp. 764-770. (jap.); PMID 9414589

