Lanthanum nitrate
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Lanthanum nitrate | |||||||||
| other names |
|
|||||||||
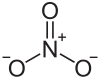
| Molecular formula | La (NO 3 ) 3 | |||||||||
| Brief description |
colorless crystalline powder with a slight odor |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 433.02 g mol −1 (hexahydrate) | |||||||||
| Physical state |
firmly |
|||||||||
| density |
|
|||||||||
| Melting point |
40 ° C |
|||||||||
| boiling point |
126 ° C (1013 hPa) (decomposition) |
|||||||||
| solubility |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Lanthanum nitrate is a chemical compound of lanthanum from the group of nitrates . The hexahydrate with the formula La (NO 3 ) 3 · 6H 2 O is always formed from an aqueous solution .
Extraction and presentation
Lanthanum nitrate can be obtained by reacting nitric acid with lanthanum , lanthanum oxide , lanthanum hydroxide or lanthanum carbonate .
properties
Lanthanum nitrate is mainly present as hexahydrate . This is a colorless crystalline powder with a slight odor that is readily soluble in water. The hexahydrate has a triclinic crystal structure with the space group P 1 (space group no. 2) . The tetrahydrate produced by the thermal decomposition of the hexahydrate occurs in two polymorphic forms, a monoclinic with the space group P 2 1 / m (space group no. 11) and an orthorhombic with the space group Pbca (space group no. 61) .
use
Lanthanum nitrate hexahydrate is used to detect an acetyl group . Solutions of lanthanum nitrate are used to detect fluoride. It also serves as a starting material for the electrochemical synthesis of LaMnO 3 thin-film coatings on stainless steel substrates.
Individual evidence
- ↑ a b c d e f g h i Data sheet lanthanum nitrate hexahydrate (PDF) from Merck , accessed on March 9, 2017.
- ^ A b R. Blachnik: Pocket book for chemists and physicists Volume 3: Elements, inorganic compounds and materials, minerals . Springer-Verlag, 2013, ISBN 978-3-642-58842-6 , pp. 528 ( limited preview in Google Book search).
- ^ Robert A. Lewis: Hawley's Condensed Chemical Dictionary . John Wiley & Sons, 2016, ISBN 978-1-119-26784-3 , pp. 1517 ( limited preview in Google Book search).
- ^ A b G. Singh: Chemistry of d-block elements . 2007, ISBN 81-8356-242-6 ( page 86 in the Google book search).
- ↑ A.-E. Gobichon, M. Louër, JP Auffrédic, D. Louër: Structure Determination of Two Polymorphic Phases of La (NO 3 ) 3 -4H 2 O from X-Ray Powder Diffraction. In: Journal of Solid State Chemistry. 126, 1996, p. 127, doi : 10.1006 / jssc.1996.0320 .
- ^ Claus Harzdorf: For the photometric titration of fluoride with lanthanum (cerium, yttrium). In: Fresenius' Journal for Analytical Chemistry. 233, 1967, p. 348, doi : 10.1007 / BF00507477 .
- ↑ Data sheet Lanthanum (III) nitrate hexahydrate, 99.999% trace metals basis from Sigma-Aldrich , accessed on May 31, 2017 ( PDF ).


