Nitrophenols
| Nitrophenols | ||||||||
| Surname | 2-nitrophenol | 3-nitrophenol | 4-nitrophenol | |||||
| other names |
o -nitrophenol, 1-hydroxy-2-nitrobenzene |
m -nitrophenol, 1-hydroxy-3-nitrobenzene |
p -nitrophenol, 1-hydroxy-4-nitrobenzene |
|||||
| Structural formula |
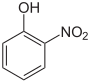
|

|
|
|||||
| CAS number | 88-75-5 | 554-84-7 | 100-02-7 | |||||
| PubChem | 6947 | 11137 | 980 | |||||
| Molecular formula | C 6 H 5 NO 3 | |||||||
| Molar mass | 139.11 g mol −1 | |||||||
| Physical state | firmly | |||||||
| Brief description |
yellowish needles or prisms |
colorless crystals |
colorless crystal needles |
|||||
| Melting point | 44 ° C | 97 ° C | 113-115 ° C | |||||
| boiling point | 214 ° C | 194 ° C (93 mbar) | Decomp. | |||||
| pK s value | 7.21 | 8.38 | 7.16 | |||||
| solubility | 2.1 g / l (20 ° C) | 13.5 g / l (25 ° C) | 14.8 g / l (25 ° C) | |||||
| Slightly soluble in water, soluble in ethanol, ether and chloroform | ||||||||
|
GHS labeling |
|
|
|
|||||
| H and P phrases | 302-312-332-410 | 302-319 | 301-312-332-373 | |||||
| no EUH phrases | no EUH phrases | no EUH phrases | ||||||
|
273-280-302 + 352 304 + 340-305 + 351 + 338-312 |
305 + 351 + 338 |
261-301 + 310-330 302 + 352-304 + 340-312 |
||||||
In chemistry, the nitrophenols form a group of substances that are derived from both phenol and nitrobenzene . The structure consists of a benzene ring with attached hydroxyl (-OH) and nitro group (-NO 2 ) as substituents . Their different arrangement results in three constitutional isomers with the empirical formula C 6 H 5 NO 3 . They are mostly accessible by nitrating phenol with (concentrated) nitric acid . Among other things, they stand out due to their yellow color.
presentation
2- and 4-nitrophenol are formed in a mixture during the nitration of phenol with dilute nitric acid. The −I effect and the + M effect of the hydroxyl group of the phenol have an ortho-para-directing effect on the second substitution. The separation is achieved by means of steam distillation , only the o -nitrophenol being transferred. New separation methods are based on the fact that only o -nitrophenol is soluble in n -pentane.
Both isomers can be further nitrated to 2,4-dinitrophenol and picric acid .
The preparation of 3-nitrophenol achieved in two stages, first by halogenation (eg Cl. 2 / AlCl 3 ) of nitrobenzene (meta-directing group); then the halogen atom is exchanged for OH in a nucleophilic aromatic substitution :
Another way is through the diazotization of 3-nitroaniline and subsequent boiling of the diazonium salt.
properties
Nitrophenols are crystalline solids; they are sparingly soluble in water, soluble in ethanol, ether and chloroform. 4-Nitrophenol has a slightly phenol-like odor.
pK s values
The weakly acidic character of phenol is due to the mesomeric stabilization of the phenolate ion. The nitro groups have an electron-withdrawing effect; the phenolic OH bond is increasingly polarized. 2- and 4-nitrophenol have a lower pK compared to the 3-nitrophenol s value; their acidities are therefore greater. In the ortho- and para-form, the phenolate ion can shift a double bond to the electron-withdrawing nitro group ( −M effect ). The second O can form a negative center of gravity there. This is not possible with the meta form.
Melting points
The melting points show clear differences. The 2-nitrophenol having the lowest melting point, as there is a intra molecular hydrogen bonds can form. In contrast, the other two isomers form intermolecular hydrogen bonds. With 2-nitrophenol, energy is not required to break these bridges. Due to its symmetry, 4-nitrophenol has the highest melting point.
solubility in water
The nitrophenols are sparingly soluble in water, but the values differ within this group. The significantly poorer solubility of 2-nitrophenol in water can also be explained well by the intramolecular hydrogen bond. As a result, the molecule is significantly less polar towards the outside. In contrast, the solubilities of 3- and 4-nitrophenol are about the same and, in comparison, significantly better. Here now form rather between the phenolic hydroxyl and water inter -molecular hydrogen bonds.
proof
For qualitative analytical proof, bromination with potassium bromide and bromine with 2-nitrophenol produces the 4,6-dibromo derivative, which has a melting point of 117 ° C. With 4-nitrophenol, the 2,6-dibromo derivative is formed with a melting point of 142 ° C. 3-Nitrophenol is also brominated twice with KBr / Br 2 ; the product melts at 91 ° C.
use
Nitrophenols occur in the chemical, pharmaceutical and defense industries as intermediate products in the production of paint, leather, rubber, pesticides, fungicides, pesticides and ammunition.
safety instructions
Nitrophenols are toxic if inhaled, swallowed and in contact with the skin. Eye, digestive tract irritation, blood poisoning, liver damage, dizziness, nausea, headache, and respiratory tract irritation may occur. They may have a carcinogenic and sensitizing effect. The effect is increased in connection with alcohol. If it comes into contact with the skin, wash off immediately with plenty of water. They have toxic effects on the nervous system of living things.
The removal of nitrophenols and related compounds from the groundwater is a major problem. Such contamination can sometimes be found in former explosives or paint factories and military installations. A new development for the complete removal of nitrophenols from groundwater is a catalyst that consists of iron with a tetra-amido- macrocyclic ligand (Fe-TAML) and enables the toxins to be oxidized by hydrogen peroxide . There are no other toxic substances. The catalyst was developed at Carnegie Mellon University.
See also
literature
- Allinger , Cava , de Jongh , Johnson , Lebel , Stevens : Organic Chemistry. Walter de Gruyter, Berlin 1980, ISBN 3-11-004594-X , pp. 375-376, 916.
- Streitwieser, Heathcock: Organic Chemistry. VCH, Weinheim 1980, ISBN 3-527-25810-8 , pp. 1056, 1179, 1189-1191.
- Hans Beyer , Wolfgang Walter : Textbook of organic chemistry. 19th edition, S. Hirzel Verlag, Stuttgart 1981, ISBN 3-7776-0356-2 , pp. 467-468.
- Morrison, Boyd: Textbook of Organic Chemistry. 3rd edition, VCH, Weinheim 1986, ISBN 3-527-26067-6 , pp. 1074-1076, 1121-1122.
Web links
Individual evidence
- ↑ a b c d e Entry on 2-nitrophenol in the GESTIS substance database of the IFA , accessed on March 12, 2017(JavaScript required) .
- ↑ a b c d e Entry on 3-nitrophenol in the GESTIS substance database of the IFA , accessed on March 12, 2017(JavaScript required) .
- ↑ a b c d e Entry on 4-nitrophenol in the GESTIS substance database of the IFA , accessed on March 12, 2017(JavaScript required) .
- ↑ CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ Test specification : Nitration of phenol to 2-nitrophenol and 4-nitrophenol (PDF) from the collection of integrated organic-chemical internship at the University of Regensburg, accessed on October 30, 2011.
- ↑ A. Khazaei, MA Zolfigol, AR Moosavi-Zare, A. Zare: An Efficient Method for the Nitration of Phenols with NaNO 2 in the Presence of 3-Methyl-1-Sulfonic Acid Imidazolium Chloride . In: Scientia Iranica . tape 17 , no. 1 , 2010, p. 31–36 ( PDF - free full text).
- ↑ Heinz GO Becker (Ed.): Organikum . 19th edition. Johann Ambrosius Barth, Deutscher Verlag der Wissenschaften, Leipzig / Berlin / Heidelberg 1993, ISBN 3-335-00343-8 , p. 331 .
- ↑ F. Wild: Characterization of Organic Compounds . CUP Archives, Cambridge 1947, p. 84 ( limited preview in Google Book search).
- ↑ F. Wild: Characterization of Organic Compounds . CUP Archives, Cambridge 1947, p. 86 ( limited preview in Google Book search).
- ↑ Heinz GO Becker (Ed.): Organikum . 19th edition. Johann Ambrosius Barth, Deutscher Verlag der Wissenschaften, Leipzig / Berlin / Heidelberg 1993, ISBN 3-335-00343-8 , p. 653 .
- ↑ Catalyst breaks down nitrophenol. In: Welt online . August 30, 2005; Retrieved February 25, 2008.
- ↑ Soumen Kundu, Arani Chanda, Jasper VK Thompson, George Diabes, Sushil K. Khetan, Alexander D. Ryabov, Terrence J. Collins: Rapid degradation of oxidation resistant nitrophenols by TAML activator and H 2 O 2 . In: Catalysis Science & Technology . tape 5 , no. 3 , 2015, p. 1775-1782 , doi : 10.1039 / C4CY01426J .









