Nitro-substituted phenols
In chemistry, nitro- substituted phenols form a larger group of aromatic nitro compounds that are derived from phenol . They are mostly accessible by nitrating phenol with (concentrated) nitric acid . Many compounds stand out because of their intense yellow color. Depending on the number of nitro groups, a distinction is made between (mono-) nitrophenols , of which there are three constitutional isomers , dinitrophenols with six isomeric compounds, of which 2,4-dinitrophenol is mainly of practical importance. Among the trinitrophenols , the most important representative is 2,4,6-trinitrophenol with the common name picric acid .
Substance groups
Depending on the degree of substitution with nitro groups, a distinction is made between:
- (Mono-) nitrophenols (three isomeric compounds), C 6 H 5 NO 3
- Dinitrophenols (six isomeric compounds), C 6 H 4 N 2 O 5
- Trinitrophenols (six isomeric compounds), C 6 H 3 N 3 O 7
- Tetranitrophenols (three isomeric compounds), C 6 H 2 N 4 O 9
- Pentanitrophenol, C 6 HN 5 O 11
properties
Acidity
| various nitrophenols and their pK s values | ||||
| Surname | phenol | 4-nitrophenol | 2,4-dinitrophenol | Picric acid |
| Structural formula |  |

|
||
| pK s value | 9.99 | 7.16 | 4.09 | 0.29 |
The weakly acidic character of the phenol is due to the mesomeric stabilization of the phenolate ion. The nitro groups have an electron-withdrawing effect; the phenolic OH bond is increasingly polarized. As the degree of nitration increases, the acid strength increases significantly. At the top is 2,4,6-trinitrophenol, it bears the common name picric acid and has an acidity in the order of magnitude of mineral acids .
safety instructions
Nitrophenols are toxic if inhaled, swallowed and in contact with the skin. Eye, digestive tract irritation, blood poisoning, liver damage, dizziness, nausea, headache, and respiratory tract irritation may occur. They may have a carcinogenic and sensitizing effect. The effect is increased in connection with alcohol. If it comes into contact with the skin, wash off immediately with plenty of water. They have toxic effects on the nervous system of living things.
Nitrophenols occur in the chemical, pharmaceutical and defense industries as intermediate products in the production of paint, leather, rubber, pesticides, fungicides, pesticides and ammunition.
The removal of nitrophenols and related compounds from the groundwater is a major problem. Such contamination can sometimes be found in former explosives or paint factories and military installations. A new development for the complete removal of nitrophenols from groundwater is a catalyst that consists of iron with a tetra-amido- macrocyclic ligand (Fe-TAML) and enables the toxins to be oxidized by hydrogen peroxide . There are no other toxic substances. The catalyst was developed at Carnegie Mellon University.
Nitrophenols
The nitrophenols form a group of compounds having a hydroxyl group and a nitro group . There are three different constitutional isomers .
| Nitrophenols | |||
| Surname | 2-nitrophenol | 3-nitrophenol | 4-nitrophenol |
| other names | o -nitrophenol | m -nitrophenol | p -nitrophenol |
| Structural formula | 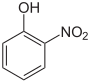 |
 |
|
| Molecular formula : C 6 H 5 NO 3 ; Molar mass : 139.11 g mol −1 | |||
Dinitrophenols
The dinitrophenols form a group of compounds with one hydroxyl group and two nitro groups . There are six different constitutional isomers , of which 2,4-dinitrophenol is the most important. The latter is formed from 2- and 4-nitrophenol by renewed nitration.
| Dinitrophenols | ||||||
| Surname | 2,3-dinitrophenol | 2,4-dinitrophenol | 2,5-dinitrophenol | 2,6-dinitrophenol | 3,4-dinitrophenol | 3,5-dinitrophenol |
| Structural formula |

|

|
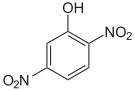
|

|

|
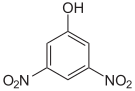
|
| Molecular formula : C 6 H 4 N 2 O 5 ; Molar mass = 184.11 g mol −1 | ||||||
Trinitrophenols
The trinitrophenols form a group of compounds with one hydroxyl group and three nitro groups . There are six different constitutional isomers , of which 2,4,6-trinitrophenol (picric acid) is by far the most important.
Picric acid forms yellow, strongly bitter-tasting crystals. Due to the accumulation of electron-withdrawing nitro (-NO 2 ), the picric acid through its phenolic hydroxy group is a strong acid (pK s = 0.29). Picric acid is produced by sulfonating phenol and then treating it with nitric acid. The use of picric acid as a filling material for grenades (as in World War I ) or generally as an explosive was discontinued due to the uncontrolled formation of highly explosive heavy metal picrates. The picric acid has been replaced by TNT here .
| Trinitrophenols | ||||||
| Surname | 2,3,4-trinitrophenol |
2,3,5-trinitrophenol |
2,3,6-trinitrophenol |
2,4,5-trinitrophenol |
2,4,6-trinitrophenol (picric acid) |
3,4,5-trinitrophenol |
| Structural formula |

|
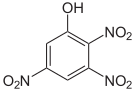
|

|

|

|

|
| Molecular formula : C 6 H 3 N 3 O 7 ; Molar mass = 229.11 g mol −1 | ||||||
Tetra- and pentanitrophenols
The 2,3,4,6-tetranitrophenol (C 6 H 2 N 4 O 9 ) is so explosive that it must not be transported. Pentanitrophenol (C 6 HN 5 O 11 ) is a hydrolysis product of hexanitrobenzene .
| Tetra- and pentanitrophenols | ||||
| Surname | 2,3,4,5-tetranitrophenol | 2,3,4,6-tetranitrophenol | 2,3,5,6-tetranitrophenol | Pentanitrophenol |
| Structural formula | 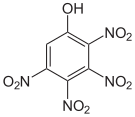 |
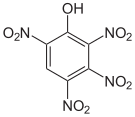 |
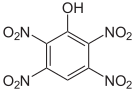 |

|
| Molecular formula | C 6 H 2 N 4 O 9 | C 6 HN 5 O 11 | ||
| Molar mass | 274.10 g mol −1 | 319.10 g mol −1 | ||
| CAS number | 641-16-7 | |||
| PubChem | 21523193 | 61190 | ||
Individual evidence
- ↑ a b Zvi Rappoport (Ed.): CRC Handbook of Tables for Organic Compound Identification . 3 rd Edition, CRC Press / Taylor and Francis, Boca Raton, FL, 1967, ISBN 0-8493-0303-6 , Acid Dissociation Constants of Phenols in Aqueous Solution, S. 434th
- ↑ WELT online: Catalyst breaks down nitrophenol (August 30, 2005) , accessed on February 25, 2008.
literature
- Allinger , Cava , de Jongh , Johnson , Lebel , Stevens : Organische Chemie , 1st edition, Walter de Gruyter, Berlin 1980, ISBN 3-11-004594-X , pp. 375-376, 916.
- Streitwieser / Heathcock: Organic Chemistry , 1st Edition, VCH, Weinheim 1980, ISBN 3-527-25810-8 , pp. 1056, 1179, 1189-1191.
- Hans Beyer and Wolfgang Walter : Textbook of Organic Chemistry , 19th edition, S. Hirzel Verlag, Stuttgart 1981, ISBN 3-7776-0356-2 , pp. 467-468.
- Morrison / Boyd: Textbook of Organic Chemistry , 3rd Edition, VCH, Weinheim 1986, ISBN 3-527-26067-6 , pp. 1074-1076, 1121-1122.

