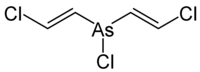
Lewisite
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| trans and cis isomers | ||||||||||
| General | ||||||||||
| Surname | Lewisite | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 2 H 2 AsCl 3 | |||||||||
| Brief description |
oily, colorless and odorless liquid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 207.32 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| density |
|
|||||||||
| Melting point |
|
|||||||||
| boiling point |
|
|||||||||
| Vapor pressure | ||||||||||
| solubility |
|
|||||||||
| Refractive index |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||
Lewisite , including lewisite I called, is a chlorine-containing organic arsenic compound whose effect when used as a chemical weapon to mustards similar. The substance causes severe burning of the skin with blistering. Lewisite was named after the American chemist Winford Lee Lewis (1878-1943), who rediscovered it after he had been made aware of the dissertation of Julius Arthur Nieuwland (1878-1936). It was nicknamed the rope of death among soldiers .
There are two configurational isomers, the trans -Lewisite and the cis -Lewisite (also called Isolewisite) .
presentation
Winford Lee Lewis produced the substance in 1918 by reacting arsenic trichloride (AsCl 3 ) with ethyne (acetylene) in the presence of hydrogen chloride in a mercury (II) chloride solution (HgCl 2 ):
Β-Lewisite (2,2'-dichlorodivinylarsine chloride) and γ-Lewisite (1,1 ', 1' '-trichlorotrivinylarsine) are also formed as by-products.
history
The substance was developed, produced and also shipped to Europe during World War I, but was no longer used. The substance was later specifically developed and manufactured by the US Army as a chemical warfare agent. Between the two world wars, it was also produced in the Soviet Union and Japan. Sometimes it was used as a poison gas mixture together with mustard gas as Mustard Lewisite for a faster effect and to lower the freezing point of the mustard gas. Lewisite is more volatile than mustard gas and is more sensitive to moisture, but acts faster and more directly than mustard gas. A total of around 20,000 tons of Lewisite were produced. Its use was discontinued at the end of the 1950s. Japan also produced lewisite during World War II and buried stocks in occupied China that were rediscovered in 2006. There is no indication of a combat mission.
properties
The cis and trans isomers or the mixture of isomers are oily, colorless and odorless liquids that decompose on contact with water. The vapors are much heavier than air. The isomers differ greatly in terms of their physical properties. The cis isomer has a much lower melting and boiling point. It is more volatile than the trans isomer because of its higher vapor pressure . The vapor pressure curve for the cis isomer results from lgp = 8.4131 - 2450.2 / T, that of the trans isomer from lgp = 48660 - 13297 lgT - 4815.3 / T (p in Torr, T in K) .
Toxic effect and antidote
The poison can be absorbed through the skin, respiratory tract and gastrointestinal tract . Lewisite reacts with the thiol groups of proteins and, as far as enzymes are concerned, massively disrupts the metabolism. Similar to the mustard, it also reacts with DNA molecules by alkylating them. Lewisite immediately produces a strong burning sensation on the skin, after 30 minutes erythema ; after 12 hours, it turns into sharply demarcated, superficial blisters up to deep painful necroses .
Dithioglycerin (other names are BAL , British Anti Lewisite , Dimercaprol or 2,3-Dimercaptopropanol) can be used as an antidote . Dithioglycerin binds Lewisite or releases Lewisite bound to proteins again.
International controls
Lewisites are listed as chemicals on List 1 of the International Chemical Weapons Convention (also Chemical Weapons Convention , CWC ) controlled by the responsible authority OPCW based in The Hague. Development or possession for purposes other than research in defense against these substances is prohibited. In Germany, any handling of List 1 chemicals - except in the business area of the Federal Ministry of Defense - must be approved by the Federal Office of Economics and Export Control (BAFA) and, depending on the activity carried out, be reported to the OPCW.
See also
Web links
- Haas, R. et al. : Chemical-analytical investigation of arsenic agents and their metabolites, UWSF - Z Umweltchem Ökotox ; 10: 289-293 (1998); PDF (free full text access)
Individual evidence
- ↑ a b c d e Entry on chlorovinyl dichloro arsine in the GESTIS substance database of the IFA , accessed on February 16, 2017(JavaScript required) .
- ↑ a b c d e f Whiting, GH: Some physicochemical properties of cis-2-chlorovinyldichloroarsine in J. Chem. Soc. 1948, 1209-1210, doi : 10.1039 / JR9480001209 .
- ↑ Lebedev, BV; Kulagina, TG; Cheremukhina, AA; Karataev, EN: Russian J. Gen. Chem. 66 (1996) 880-885.
- ↑ a b c d e f Entry on Lewisite. In: Römpp Online . Georg Thieme Verlag, accessed on November 5, 2011.
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry arsenic compounds, with the exception of those named in this appendix in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA) on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b Entry on Lewisite in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ External identifiers or database links for 2,2'-dichlorodivinylarsine chloride: CAS number: 40334-69-8, PubChem : 5368106 , Wikidata : Q55690543 .
- ↑ External identifiers of or database links to 1,1 ', 1' '- trichlorotrivinylarsine : CAS number: 40334-70-1, PubChem : 5352143 , Wikidata : Q55690530 .
- ^ Robin Black, Development, Historical Use and Properties of Chemical Warfare Agents, in: Franz Worek u. a. (Ed.), Chemical Warfare Technology, Royal Society of Chemistry, 2016, Volume 1, p. 16
- ↑ Domingo Tabangcura, Jr. and G. Patrick Daubert, MD. British anti-Lewisite Development Molecule of the Month , University of Bristol School of Chemistry.
- ↑ a b Worek u. a. (Ed.), Chemical Warfare Technology, 2016, Volume 1, p. 16.
- ^ NTI: Abandoned Chemical Weapons (ACW) in China ( Memento of September 29, 2011 in the Internet Archive ).
- ↑ a b Wissenschaft-online: Entry on Lewisite in the lexicon of biology / chemistry .
- ↑ According to other information, bubbles form in a few hours. Franz Worek u. a. (Ed.), Chemical Warfare Technology, 2016, Volume 1, p. 16
- ↑ Chemicals on list 1 at the Federal Office of Economics and Export Control (BAFA), accessed on January 15, 2013.
- ↑ Chemical Weapons Convention: Information on the Chemical Weapons Convention (CWÜ) at the Federal Office of Economics and Export Control (BAFA), accessed on January 15, 2013.


![{\ displaystyle \ mathrm {AsCl_ {3} + HC {\ equiv} CH \ {\ xrightarrow [{HCl}] {HgCl_ {2}}} \ Cl_ {2} As {-} CH {=} CHCl}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/22931ff390e49dc51c719f761e8a4c4c351acfe3)