Amatoxins

The amatoxins are cyclic oligopeptides , circular compounds made up of eight amino acids . There are at least ten amatoxins, of which α-amanitin, β-amanitin and γ-amanitin are the best known. Amatoxins come alongside the similarly constructed Phallotoxinen in some species of the genus Amanita ( Amanita before), for example in the European representatives Amanita phalloides ( A. phalloides ), Amanita virosa ( A. virosa ) and Spring Knollenblätterpilz ( A. verna ). Various types of mushrooms from the genera of the capes ( Galerina ), the umbrella ( Lepiota ) and the velvet cap ( Conocybe ) contain amatoxins.
properties
The amatoxins are resistant to cooking and drying; the eight amino acid peptides are not broken down by the proteases in the gastric tract. The reason for this lies in the special structure of these bicyclic octapeptides.
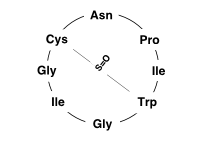
The ring closure of the oligopeptide chain makes it cyclopeptides and a special additional internal cross-link between its amino acids tryptophan and cysteine makes it a bicyclic peptide. This Trp - Cys cross bridge as sulfoxide is a characteristic structural feature of the amatoxins and essential for their extremely high tendency to bind to central regions of certain RNA polymerases , which are necessary for transcription in animal cells . When they are blocked, protein synthesis comes to a standstill. The RNA polymerase of the fungal species itself is insensitive to this.
A similar bridging of Trp - Cys via a sulfur atom characterizes - here as a sulfide or thioether - also the closely related bicyclic heptapeptides of the phallotoxins , which bind to F-actin with high affinity . Such cross bridges through an additional internal link between cysteine and tryptophan, also called tryptathionine , have not been found in other natural products so far.
Amatoxins, which include amanitins, contain only L- amino acids as building blocks, some of which are hydroxylated . In the case of α-Amanitin , in addition to hydroxyproline and 6-hydroxytryptophan , these are also a bishydroxylated isoleucine - the (4 R ) -4,5-dihydroxy- L- isoleucine was first discovered here as a peptide building block - as well as isoleucine, cysteine, glycine (twice) and asparagine . In its place, β-Amanitin has aspartic acid , γ-Amanitin uses hydroxyisoleucine instead of dihydroxyisoleucine, and ε-Amanitin both is the case. The structure of δ-aminitine is not clear.
α-Amanitin has the empirical formula C 39 H 54 N 10 O 14 S, a molar mass of 918.98 g / mol and the CAS number 23109-05-9.
β-Amanitin has the empirical formula C 39 H 53 N 9 O 15 S, a molar mass of 919.97 g / mol and the CAS number 21150-22-1.
γ-Amanitin has the empirical formula C 39 H 54 N 10 O 13 S, a molar mass of 902.98 g / mol and the CAS number 21150-23-2.
ε-Amanitin has the empirical formula C 39 H 53 N 9 O 14 S, a molar mass of 903.97 g / mol and the CAS number 21705-02-2.
All four are crystalline, colorless, heat-stable and soluble in ethanol , methanol and water.
Structural similarity
| Surname | R 1 | R 2 | R 3 | R 4 | R 5 |
|---|---|---|---|---|---|
| Amanine | OH | OH | OH | H | OH |
| Amaninamide | OH | OH | NH 2 | H | OH |
| α-amanitin | OH | OH | NH 2 | OH | OH |
| β-amanitin | OH | OH | OH | OH | OH |
| γ-amanitin | H | OH | NH 2 | OH | OH |
| ε-amanitin | H | OH | OH | OH | OH |
| Amanullic acid | H | H | OH | OH | OH |
| Amanulline | H | H | NH 2 | OH | OH |
| Proamanullin | H | H | NH 2 | OH | H |

|
1 | 2 | 3 | 4th | 5 | 6th | 7th | 8th | LD 50 (mg / kg) oral, mouse |
|---|---|---|---|---|---|---|---|---|---|
| Amanine | Dihyile | Trp | Gly | Ile | Gly | Cys | Asp | Hypro | 0.5 |
| Amaninamide | Dihyile | Trp | Gly | Ile | Gly | Cys | Asn | Hypro | 0.5 |
| α-amanitin | Dihyile | Hytrp | Gly | Ile | Gly | Cys | Asn | Hypro | 0.3 |
| β-amanitin | Dihyile | Hytrp | Gly | Ile | Gly | Cys | Asp | Hypro | 0.5 |
| γ-amanitin | Hyile | Hytrp | Gly | Ile | Gly | Cys | Asn | Hypro | 0.2 |
| ε-amanitin | Hyile | Hytrp | Gly | Ile | Gly | Cys | Asp | Hypro | 0.3 |
| Amanullic acid | Ile | Hytrp | Gly | Ile | Gly | Cys | Asp | Hypro | > 20 |
| Amanulline | Ile | Hytrp | Gly | Ile | Gly | Cys | Asn | Hypro | > 20 |
| Proamanullin | Ile | Hytrp | Gly | Ile | Gly | Cys | Asn | Per | > 20 |
|
|||||||||
biosynthesis
Small cyclic peptides such as amanitin are commonly synthesized in fungi as non-ribosomal peptides by non-ribosomal peptide synthetases (NRPS). After the genome of the North American amanita bisporigera was completely sequenced, no genes coding for the corresponding NRPS were found , which ruled out this possibility here. However, the search for a possible coding sequence that indicates the types of amino acids contained in Amanitin (Ile, Trp, Gly, Cys, Asn, Pro) in eight positions in the correct order led to a section in a gene which was called AMA1 . The proprotein encoded by AMA1 is, however, with 35 amino acids much longer than the finished Amanitin with 8 AA , so it has to be shortened post-translationally by a peptidase . Further investigations showed that the section with the amino acid sequence I W G IG C N P is cut out of the polypeptide in a first step by a prolyl oligopeptidase (POP). The further processing - which includes a cyclization of the peptide, its internal bridging and the hydroxylation of individual amino acids - is not yet known in detail.
toxicity

Amatoxins inhibit transcription by blocking RNA polymerases . This mechanism is selective and starts with specific structural features of the RNA polymerases; Thus those of the Amanita mushroom species themselves are not affected, whereas RNA polymerase II from mammalian species in particular is affected . Due to the failure of the mRNA synthesis, their blockage means that genetic information can no longer pass from the cell nucleus into the cell plasma , where protein biosynthesis (as translation ) takes place.
The different RNA polymerases of mammalian cells are differently sensitive to Amanitin. The RNA polymerase II, which is necessary for mRNA synthesis , among other things, is severely affected . The inhibition of RNA polymerase III, which is required for tRNA synthesis, is markedly weaker, and RNA polymerase I, which is needed for rRNA synthesis, is not affected. Because of the diverse functions of the different proteins formed after an mRNA is presented , numerous processes of the organism are affected:
- Enzymes are no longer formed and the metabolic processes they catalyze come to a standstill.
- Structural proteins are no longer replaced with aging.
- Hormones (both peptide and enzymatically produced hormones) no longer contribute to the control of metabolic processes.
- Membrane receptors , for example on nerve cells, are not reproduced.
In the case of poisoning by death cap mushrooms, the effects initially appear as diarrhea , which is triggered by damage to the epithelial cells of the intestine. Only later - after the toxins have been absorbed into the blood, their distribution in the body, their absorption in cells and the binding to RNA polymerase complexes in the cell nucleus - do further symptoms occur, which are based on irreversible cell damage. A sufficiently high dose can also lead to liver failure , which is fatal. The toxic effect of the Amanitine is intensified by their enterohepatic circulation ; the Amanitin circulates between the liver, gall bladder and intestine and thus remains in the body longer.
Antidote
The only known antidote is silibinin .
Applications
Many tumors that have gene for the tumor suppressor p53 lost , and often an adjoining gene copy for the biggest and catalytically active subunit of RNA polymerase II (POLR2A) is also lost. The inhibition of RNA polymerase II with α-Amanitin is therefore being investigated for the treatment of p53-negative tumors, since the tumor cells suffer more from the inhibition than healthy cells.
history
After Feodor Lynen and Ulrich Wieland were able to produce a phallotoxin phalloidin , a phallotoxin , in crystalline form as early as 1937 , amatoxins were isolated for the first time in 1941 by Heinrich Wieland and Rudolf Hallermayer as the “amanitin” toxin of the tuber fungus. The individual components of the toxin mixture were later differentiated by Theodor Wieland and co-workers as α- , β- and γ-Amanitin and their structure was clarified in 1966.
literature
- Entry to Amanitine. In: Römpp Online . Georg Thieme Verlag, accessed on June 7, 2014.
- Theodor Wieland : Amatoxins, phallotoxins - the poisons of the death cap mushroom . In: Chemistry in our time , 13th year 1979, No. 2, pp. 56-63.
Individual evidence
- ↑ a b c J. Vetter: Toxins of Amanita phalloides. In: Toxicon: official journal of the International Society on Toxinology. Volume 36, Number 1, January 1998, pp. 13-24, PMID 9604278 .
- ↑ J. May, D. Perrin: Tryptathionine bridges in peptide synthesis . In: Biopolymers . 88, No. 5, 2007, pp. 714-724. PMID 17626299 .
- ↑ Jonathan Walton, Heather Hallen-Adams, Hong Luo: Ribosomal Biosynthesis of the Cyclic Peptide Toxins of Amanita Mushrooms. In: Biopolymers , Volume 94, Number 5, 2010, pp. 659-664, PMC 4001729 (free full text).
- ↑ David G. Spoerke, Barry H. Rumack: Handbook of mushroom poisoning: diagnosis and treatment. CRC Press, 1994. ISBN 0-8493-0194-7 , p. 167 ff.
- ↑ a b UniProt P85421 (α-, γ-Amanitin).
- ↑ Heather E. Hallen: Gene family encoding the major toxins of lethal Amanita mushrooms . In: Proceedings of the National Academy of Sciences , Vol. 104, 2007, no.48.
- ^ H. Luo, HE Hallen-Adams, JD Walton: Processing of the phalloidin proprotein by prolyl oligopeptidase from the mushroom Conocybe albipes. In: The Journal of biological chemistry. Volume 284, Number 27, July 2009, pp. 18070-18077, doi: 10.1074 / jbc.M109.006460 , PMID 19389704 , PMC 2709354 (free full text).
- ↑ F. Brueckner, P. Cramer: Structural basis of transcription inhibition by alpha-amanitin and implications for RNA polymerase II translocation. In: Nature Structural & Molecular Biology. Volume 15, August 2008, pp. 811-818, PMID 18552824 .
- ↑ Th. Zilker, JJ Kleber, B. Haber: Amatoxinsyndrom . toxinfo.org, 2000
- ↑ a b Yunhua Liu, Xinna Zhang, Cecil Han, Guohui Wan, Xingxu Huang, Cristina Ivan, Dahai Jiang, Cristian Rodriguez-Aguayo, Gabriel Lopez-Berestein, Pulivarthi H. Rao, Dipen M. Maru, Andreas Pahl, Xiaoming He, Anil K. Sood, Lee M. Ellis, Jan Anderl, Xiongbin Lu: TP53 loss creates therapeutic vulnerability incolorectal cancer. In: Nature. 520, 2015, p. 697, doi: 10.1038 / nature14418 .
- ↑ H. Wieland, R. Hallermayer: About the toxins of the death cap mushroom. VI. Amanitin, the main poison of the death cap mushroom . In: Justus Liebig's Annals of Chemistry . Volume 348, No. 1, May 1941, pp. 1-18. doi : 10.1002 / jlac.19415480102 .
- ↑ T. Wieland, U. Gebert: About the ingredients of the green leaf mushroom, XXX. Amanitine structures . In: Justus Liebig's Annals of Chemistry . Volume 700, No. 1, January 1967, pp. 157-173. doi : 10.1002 / jlac.19667000119 .