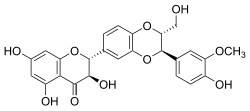
Silibinin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Silibinin | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 25 H 22 O 10 | |||||||||||||||||||||
| Brief description |
colorless crystals |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 482.44 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
167 ° C (as monohydrate) |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Silibinin , also called silybin , is the most strongly pharmacologically active substance of the complex silymarin , which is obtained from the fruits of the milk thistle ( Silybum marianum ). It is considered to be the lead substance for medicinally used milk thistle fruit extracts, the active ingredient content of which is standardized to silibinin. Milk thistle fruit extract is said to protect the liver and strengthen the liver's function .
Description and mechanisms of action
From a chemical point of view, silibinin is a mixture of diastereomers consisting of silybin A and silybin B, which differ in their configuration at the C-2 ″ and C-3 ″.
An isolated silibinin is in the treatment of liver poisoning by toxins of Amanita mushroom ( amatoxins ) such as amanitin and phalloidin used. For this purpose, silibinin is partially combined with a β-lactam antibiotic that is supposed to support the antidotic effect. The water-soluble disodium salt of the succinic acid ester of silibinin, disodium silibinin- C -2 ', 3-bis (hydrogen succinate), is used pharmaceutically , since the therapy is carried out by means of intravenous administration. The liver-protecting effect is due to the cell membrane-stabilizing properties of silibinin. The absorption of amatoxins into the liver cells is made more difficult and their enterohepatic circulation is prevented. Silibinin intervenes in the transport mechanisms of the hepatocytes by inhibiting the reuptake of amatoxins . It specifically inhibits the organic anion transporter OATP1B3 (= SLCO1B3). A second mechanism of action is the inhibition of the release of TNF-α .
Since the introduction of therapy for amanitin-related fungal poisoning, the mortality rate has decreased to 5 to 12% with timely treatment, previously it was 20 to 30%.
Successes in the treatment of prostate cancer cells could also be shown in the laboratory, which is justified with an inhibition of CD44 expression.
Studies have shown that it similar to allopurinol , the xanthine oxidase inhibiting and thus hyperuricemia and consequent gout can counteract.
Silibinin has also been researched as a new, non-invasive treatment strategy for Cushing's disease for several years.
Trade names
Legalon ® SIL ( Madaus ) (D, CH, A)
Silimarit ® ( Bionorica ), a silymarin product
literature
- K. Letschert, H. Faulstich a. a .: Molecular characterization and inhibition of amanitin uptake into human hepatocytes. In: Toxicological Sciences . Volume 91, Number 1, May 2006, pp. 140-149, doi : 10.1093 / toxsci / kfj141 . PMID 16495352 .
- R. Agarwal: Cell signaling and regulators of cell cycle as molecular targets for prostate cancer prevention by dietary agents. In: Biochemical Pharmacology . Volume 60, Number 8, October 2000, pp. 1051-1059, PMID 11007941 . (Review).
- X. Zi, R. Agarwal: Silibinin decreases prostate-specific antigen with cell growth inhibition via G1 arrest, leading to differentiation of prostate carcinoma cells: implications for prostate cancer intervention. In: PNAS . Volume 96, Number 13, June 1999, pp. 7490-7495, PMID 10377442 . PMC 22113 (free full text).
- Mathias Riebold et al .: A C-terminal HSP90 inhibitor restores glucocorticoid sensitivity and relieves a mouse allograft model of Cushing disease. In: Nature Medicine , volume 21, pp. 276-280 (2015) doi : 10.1038 / nm.3776
Individual evidence
- ↑ a b c d Entry on Silybin. In: Römpp Online . Georg Thieme Verlag, accessed on June 21, 2014.
- ↑ a b Datasheet Silibinin from Sigma-Aldrich , accessed on April 23, 2011 ( PDF ).
- ↑ Technical information from the Swiss drug compendium for Legalon ® SIL by Max Zeller Söhne - as of June 2007.
- ↑ a b U. Mengs, RT Pohl, T. Mitchell: Legalon® SIL: the antidote of choice in patients with acute hepatotoxicity from amatoxin poisoning. In: Current Pharmaceutical Biotechnology . Volume 13, Number 10, August 2012, pp. 1964-1970, PMID 22352731 . PMC 3414726 (free full text). (Review).
- ↑ L. Al-Anati, E. Essid et al. a .: Silibinin protects OTA-mediated TNF-alpha release from perfused rat livers and isolated rat Kupffer cells. In: Molecular nutrition & food research. Volume 53, number 4, April 2009, pp. 460-466, doi : 10.1002 / mnfr.200800110 . PMID 19156713 .
- ↑ AM Handorean, K. Yang, EW Robbins, TW Flaig, KA Iczkowski: Silibinin suppresses CD44 expression in prostate cancer cells. In: American Journal of Translational Research . Volume 1, Number 1, 2009, pp. 80-86, PMID 19966941 , PMC 2776293 (free full text).
- ↑ JM Pauff, R. Hille: Inhibition studies of bovine xanthine oxidase by luteolin, silibinin, quercetin, and curcumin. In: Journal of natural products. Volume 72, number 4, April 2009, pp. 725-731, doi : 10.1021 / np8007123 , PMID 19388706 , PMC 2673521 (free full text).
- ↑ Max Planck Society : Silibinin - plant-based active ingredient against brain tumors. February 9, 2015.
- ^ Max Planck Society: IBI licenses Silibinin for the treatment of Cushing's disease. July 10, 2018
- ↑ Silibinin: New Approach to Cushing's Disease? In: Pharmazeutische Zeitung , July 12, 2018.
