Coumarin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Coumarin | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 9 H 6 O 2 | |||||||||||||||||||||
| Brief description |
colorless, burning tasting prisms, with a sweet, spicy, hay-like smell |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 146.14 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
0.94 g cm −3 (20 ° C) |
|||||||||||||||||||||
| Melting point |
68-71 ° C |
|||||||||||||||||||||
| boiling point |
298-302 ° C |
|||||||||||||||||||||
| Vapor pressure |
1.3 hPa (at 106 ° C) |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Coumarin or coumarin is a naturally occurring, aromatic secondary plant substance from the group of benzopyrones with a peculiar, pleasantly spicy odor. If it is ingested in large quantities, it is harmful to health. Compounds that contain the structural framework of coumarin are also listed under the collective name coumarins ; The derivatives of 4-hydroxycoumarins , as 4-hydroxycoumarins, are important as anti-coagulant drugs and pesticides. The name is derived from the Spanish Tupí word cumarú , tonka bean tree .
Occurrence
Coumarin is found in various stalks and butterflies , for example the yellow sweet clover ( Melilotus officinalis ), in the woodruff ( Galium odoratum ), the stone sissy ( Prunus mahaleb ), in dates as well as in the tonka bean ( Dipteryx odorata ) and in the cinnamon varieties Cassia cinnamon ( Cinnamomum) cassia ), Indonesian cinnamon ( Cinnamomum burmannii ) and Vietnamese cinnamon ( Cinnamomum loureiroi ).
Coumarin (and related substances) are responsible for the typical smell of hay when drying grass or woodruff. In the plant, coumarin is partially bound glycosidically and is only released by splitting off the sugar when the plants are injured or wither. If the glycoside and the associated glycosidase come together, for example due to the destruction or decomposition of the plant cell, the glycoside is hydrolytically split, the substance (in this case the coumarin) is released and can develop its effect.
history
Coumarin was first isolated from tonka beans in 1813 by A. Vogel from Munich, who initially thought it was benzoic acid , and by the French Jean-Baptiste-Gaston Guibourt in 1820, who recognized it as a separate substance. In 1846, H. Bleibtreu determined the correct composition and narcotic effect. The first synthetic production by William Henry Perkin succeeded in 1868 , after which it was first marketed in 1876. A breakthrough on the perfume market and the use of artificial fragrances was the perfume Fougère Royale (German: royal fern ) released in 1881 by House of Houbigant , which was based on coumarin and was very successful. Since 1954, coumarin has been banned as a flavoring substance in the USA, as toxic effects have been found in animal experiments.
In Germany, the use of coumarin as a flavor was banned by the Flavor Ordinance of December 22, 1981, later the limit value for coumarin as a food additive according to Annex 4 to Section 2, Paragraph 3 of the revised Flavor Ordinance (in the 1991 version) was currently 2 mg per kilogram of prepared food. The ban on coumarin was introduced by the Flavor Ordinance 1991 for use as a flavoring in preparations that are not used for nutrition, such as. B. in perfumes or candles. The addition of coumarin to tobacco products prohibits the tobacco regulation . In the winter of 2006/2007, coumarin made headlines in Germany when the cinnamon used in Christmas biscuits found an amount of aroma that was many times higher than the legal maximum.
synthesis
The starting material for coumarin in the plant is cinnamic acid , from which it is formed through hydroxylation, glycosidation and cyclization . The substance, in turn, is the basic body of numerous natural substances , including esculin , furocoumarins and umbelliferone .
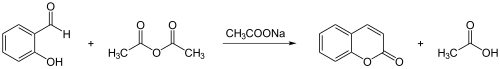
Coumarin is synthetically produced according to Perkin ( Perkin's synthesis ) from salicylaldehyde and acetic anhydride :
An alternative manufacturing process is the Raschig process from o -cresol .
Analytics
The reliable qualitative and quantitative determination of coumarin is possible after appropriate sample preparation by coupling HPLC or gas chromatography with mass spectrometry .
use
Coumarin is mainly used as a fragrance in perfumery. It is also used in the kitchen (in the form of wilted woodruff leaves ), for example to flavor May punch .
The tonka bean contains large amounts of coumarin, which is therefore often extracted from it. Because of the property of coumarin to simulate the taste of real vanilla, it has been incorrectly referred to as Mexican vanilla since the beginning of the 20th century and is used as a substitute for spiced vanilla ( Vanilla planifolia ). However, the use of coumarin as a flavoring is restricted by law in some areas (e.g. USA, European Union). In the European Union , according to Regulation (EC) No. 1334/2008, the so-called Flavor Regulation , coumarin is a substance that may not be added to food as such. If coumarin occurs naturally in flavors or food ingredients with flavor properties, certain maximum levels in the food must not be exceeded; Depending on the type of food, the maximum permissible amounts are between 5 mg / kg for dessert dishes and 50 mg / kg for traditional and / or seasonal baked goods, provided that cinnamon is specified as an ingredient on the label .
Coumarin is well absorbed through the skin. It can be used in cosmetics in Europe without limitation, but must be declared if a certain amount is exceeded.
Furthermore, coumarin as a substrate in the reaction marker for CYP 2A6 in in vitro - metabolism used. It is metabolized to 7-hydroxycoumarin ( umbelliferone ).
Coumarins are also used as photolabile protective groups .
In dye lasers find coumarin dyes as the laser medium use.
Derivatives
The anticoagulant coumarin derivatives phenprocoumon , warfarin and ethylbiscoumacetate are used in medicine for people at risk, for example, to prevent ischemic strokes . In addition, they are used as rodenticides , especially for combating rats, since in high doses they lead to fatal internal bleeding. In naturopathy, extracts from ash bark are used, the effects of which can possibly be attributed to the coumarin derivative Fraxin .
Highly fluorescent coumarin derivatives are also used as effective dyes in dye lasers and optical brighteners . As dye lasers, they emit in the blue to the green spectral range of the light spectrum.
The isocoumarin is a Stellungisomer of coumarin in which the carbonyl group and the oxygen atom are reversed. Some dihydro-isocoumarin derivatives, such as B. Phyllodulcin , which is found in the leaves of the garden hydrangea, have a sweet taste. The sweetness of phyllodulcin compared to sucrose is 250.
Dihydrocoumarin and 6-methylcoumarin are used as substitutes in industry .
Physiology and Toxicology
The kinetics are strongly species-specific. The main metabolic pathway in humans is hydroxylation at position 7 to the non-toxic umbelliferone , catalyzed by the enzyme CYP2A6 . In rats, however, the metabolism dominates via 3,4- epoxidation . In an aqueous, glutathione-free environment, the epoxide (with ring opening and decarboxylation ) quickly rearranges to form o-hydroxyphenylacetaldehyde (o-HPA), which is toxic to the liver. Its oxidation to o-hydroxyphenylacetic provides a detoxification is exceeded. The last two metabolites are detectable at low levels in humans.
Added in larger quantities causes coumarin severe headache , vomiting, dizziness and somnolence . Even higher doses can lead to central paralysis , respiratory arrest and coma . In addition, liver and kidney damage are observed in animal experiments. However, food and cosmetics containing coumarin only pose a hepatotoxic risk for humans in exceptional cases. The oral lethal dose (LD 50 ) is 293 mg / kg in rats and 202 mg / kg body weight in guinea pigs. Animal experiments lead to the suspicion that coumarin is carcinogenic in very high amounts . However, several studies on human cell lines do not indicate such effects in the human organism.
As a TDI ( tolerable daily intake , daily dose tolerated ) comes from studies of the Federal Institute for Risk Assessment (BfR) in early 2006 a quantity of 0.1 milligrams per kilogram of body weight per day out. The BfR confirmed this TDI value on the basis of new data on the uptake and bioavailability of coumarin in September 2012. At the same time, the BfR points out that the TDI value can only be exceeded if large quantities of cinnamon spices are consumed. This is possible in the run-up to Christmas when cassia cinnamon is used for baking. In infants having a body weight of 15 kg, according to the BfR TDI would be at a consumption of 6 cinnamon or 100 g gingerbread exhausted. Commercially available cinnamon rolls from various manufacturers contained 22–77 mg / kg coumarin in a 2006 study. Cinnamon capsules for diabetics are also problematic in this regard. However, there is no clear evidence of the alleged dangerousness of coumarin during normal use of spices containing coumarin. In all of the studies, adverse health effects only occurred after extreme overdoses in experiments on rats.
For the well-known Maibowle from woodruff , a maximum of 3 g of herb per liter of punch should be used. In this small amount, the contained coumarin is not harmful to the health of adults.
While coumarin itself has no anticoagulant properties, improper silo storage of grass clippings can lead to fungal infestation of coumarin-containing grasses, whereby coumarin derivatives (bis-hydroxycoumarins) are formed, which show this effect. Such contaminated feed can lead to the death of the animals fed with it, since bis-hydroxycoumarins - as antagonists of vitamin K - impair the synthesis of the blood coagulation factors (II, VII, IX, X) formed in the liver by inhibiting enzymes. In the drying process, the production of hay , on the other hand, the coumarin glycosides are converted into pure coumarin and are harmless. So serve hay flowers , the fines of hay, as a traditional remedy.
Insect repellent
Coumarin, for example in the form of fragrant Mary's grass , has a mosquito-repellent effect . In the case of sweet clover , some grasses and woodruff, coumarins released from the vacuole serve as protection against feeding.
Web links
- Manufacture of may punch and chemical background
- BfR: Questions and answers on coumarin in cinnamon and other foods ; (09/2012).
- BfR: New findings on coumarin in cinnamon (PDF; 86 kB); dated September 27, 2012.
Individual evidence
- ↑ Entry on COUMARIN in the CosIng database of the EU Commission, accessed on February 16, 2020.
- ↑ a b c d Entry on coumarin. In: Römpp Online . Georg Thieme Verlag, accessed on December 25, 2014.
- ↑ a b c d e f g Entry on coumarin in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Data coumarin (PDF) at Merck , accessed on 23 March 2011th
- ↑ Yan-Hong Wang, Bharathi Avula et al. a .: Cassia Cinnamon as a Source of Coumarin in Cinnamon-Flavored Food and Food Supplements in the United States. In: J. Agric. Food Chem. 61 (18), 2013, pp. 4470-4476, doi: 10.1021 / jf4005862 .
- ↑ D. Lowe, Das Chemiebuch, Librero 2017, p. 176
- ↑ BfR: Consumers who consume a lot of cinnamon are currently exposed to too much coumarin . Statement by the Federal Institute for Risk Assessment of June 16, 2006 (PDF; 136 kB).
- ↑ Z. Ren, B. Nie, T. Liu et al .: Simultaneous Determination of Coumarin and Its Derivatives in Tobacco Products by Liquid Chromatography-Tandem Mass Spectrometry. In: Molecules. 21 (11), 2016, pii: E1511, PMID 27834935 .
- ↑ G. Zhao, C. Peng, W. Du, S. Wang: Pharmacokinetic study of eight coumarins of Radix Angelicae Dahuricae in rats by gas chromatography-mass spectrometry. In: Fitoterapia. 89, 2013, 250-6, PMID 23774663 .
- ↑ B. Li, X. Zhang, J. Wang et al .: Simultaneous characterization of fifty coumarins from the roots of Angelica dahurica by off-line two-dimensional high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. In: Phytochem Anal. 25 (3), 2014, 229-40, PMID 24481589 .
- ↑ Juliane Daphi-Weber, Heike Raddatz, Rainer Müller: Investigation of Fragrances - Controlled Fragrances , pp. 94–95, in Volume V of the series HighChem hautnah - News from food chemistry (published by the Society of German Chemists ) 2010, ISBN 978- 3-936028-64-5 .
- ↑ European Food Safety Authority (EFSA): Opinion of the Scientific Panel on food additives, flavors, processing aids and materials in contact with food (AFC) related to Coumarin ; PDF .
- ↑ Art. 6 Paragraph 1 of Regulation (EC) No. 1334/2008 of the European Parliament and of the Council of December 16, 2008 on flavorings and certain food ingredients with flavoring properties for use in and on foods (consolidated version 2019) with Annex III, Part A. A violation, including negligent addition, constitutes in Germany according to § 58 para. 2a no. 1 LFGB constitutes a criminal offense.
- ↑ Art. 6 Paragraph 2 with Annex III Part B of Regulation (EC) No. 1334/2008 of the European Parliament and of the Council of December 16, 2008 (Flavor Regulation ).
- ↑ Takaaki Yasuda, Mai Fukui, Takahiro Nakazawa, Ayumi Hoshikawa, Keisuke Ohsawa: Metabolic Fate of Fraxin Administered Orally to Rats. In: J. Nat. Prod. 2006, 69 (5), pp. 755-757 ( doi: 10.1021 / np0580412 ).
- ↑ Entry on isocoumarins. In: Römpp Online . Georg Thieme Verlag, accessed on February 4, 2018.
- ↑ H.-D. Belitz et al .: Textbook of Food Chemistry. 5th ed., Springer, Berlin a. a. 2001, ISBN 978-3-540-41096-6 , pp. 431-432.
- ^ Dolf De Rovira, Sr .: Dictionary of Flavors. Third Edition, Wiley, 2017, ISBN 978-1-118-85641-3 , p. 80.
- ^ A b S. L. Born, D. Caudill, BJ Smith, LD Lehman-McKeeman: In vitro kinetics of coumarin 3,4-epoxidation: application to species differences in toxicity and carcinogenicity . In: Toxicol. Sci. . 58, No. 1, 2000, pp. 23-31. PMID 11053537 .
- ↑ Born SL, Caudill D, Fliter KL, Purdon MP: Identification of the cytochromes P450 that catalyze coumarin 3,4-epoxidation and 3-hydroxylation . In: Drug Metab. Dispos. . 30, No. 5, 2002, pp. 483-487. PMID 11950775 .
- ↑ LB von Weymarn, SE Murphy: CYP2A13-catalysed coumarin metabolism: comparison with CYP2A5 and CYP2A6 . In: Xenobiotica . 33, No. 1, 2003, pp. 73-81. PMID 12519695 .
- ↑ SL Born, JK Hu, LD Lehman-McKeeman: o-hydroxyphenylacetaldehyde is a hepatotoxic metabolite of coumarin . In: Drug Metab. Dispos. . 28, No. 2, 2000, pp. 218-223. PMID 10640521 .
- ↑ Rudolf Hänsel, Otto Sticher: Pharmakognosie - Phytopharmazie , Springer, Heidelberg 2009, ISBN 978-3-642-00962-4 , p. 1081.
- ↑ Weber, US. et al. (1998): Antitumor activities of coumarin. In: Res. Commun. Mol. Pathol. Pharmacol. 99 (2), pp. 193-206; PMID 9583093 .
- ↑ CM Elinos-Báez et al. (2005): Effects of coumarin and 7OH-coumarin on bcl-2 and Bax expression in two human lung cancer cell lines in vitro. In: Cell Biol. Int. 29 (8), pp. 703-708 ( PMID 15964220 ; doi: 10.1016 / j.cellbi.2005.04.003 ).
- ↑ Simon Mills, Kerry Bone: Principles and Practices of Phytotherapy . Churchill Livingstone, Edinburgh 1999, 2000, ISBN 978-0-443-06016-8 .
- ↑ K. Abraham, F. Wöhrlin u. a .: Toxicology and risk assessment of coumarin: focus on human data. In: Molecular nutrition & food research. Volume 54, number 2, February 2010, pp. 228-239, doi: 10.1002 / mnfr.200900281 . PMID 20024932 . (Review).
- ↑ Consume cassia cinnamon with high levels of coumarin in moderation - BfR .
- ↑ Health risk cannot be excluded. Pharmaceutical newspaper 44/2006.
- ↑ cf. Foin Coupe (hay smell). In: Fred Winter: Fragrances and Perfuming Technology: Genesis, Characteristics and Chemistry of Fragrances with Special Consideration of Their Practical Use for the Production of Complex Fragrance Mixtures. Springer-Verlag, 2013, ISBN 978-3-7091-5731-2 , p. 319 ff ( limited preview in the Google book search).
- ↑ Sweet-smelling secrets of mosquito-repellent grass In: BBC News. 19th August 2015.