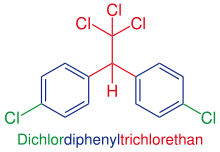
Dichlorodiphenyltrichloroethane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Dichlorodiphenyltrichloroethane | |||||||||||||||
| other names | ||||||||||||||||
| Molecular formula | C 14 H 9 Cl 5 | |||||||||||||||
| Brief description |
colorless, characteristic odor, flammable, in pure form crystals, technical product waxy |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 354.49 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.56 g cm −3 |
|||||||||||||||
| Melting point |
108.5-109 ° C |
|||||||||||||||
| boiling point |
decomposition |
|||||||||||||||
| Vapor pressure |
0.025 mPa (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
1 mg m −3 |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Dichlorodiphenyltrichloroethane , abbreviated to DDT , is an insecticide that has been used as a contact poison and food poison since the early 1940s . For decades it was the most widely used insecticide in the world because of its good effectiveness against insects, the low toxicity to mammals and the simple manufacturing process. However, due to its chemical stability and good fat solubility, it accumulated in human and animal tissues at the end of the food chain .
Over time, DDT and some of its breakdown products have been found to exhibit hormone-like effects . Birds of prey laid eggs with thinner shells, which resulted in significant populations. DDT has been suspected of causing cancer in humans. For these reasons, the use of DDT was banned by most of the western industrialized countries in the 1970s. In countries that ratified the Stockholm Convention from 2004, the manufacture and use of DDT is only permitted for the control of disease-transmitting insects , in particular the carriers of malaria .
history
discovery
DDT was first synthesized in 1874 by the Austrian chemist Othmar Zeidler under the direction of Adolf von Baeyer . The insecticidal effect was not discovered until 1939 by the Swiss Paul Hermann Müller , who received the Nobel Prize in Medicine for this in 1948 . Müller was a member of a research group at the J. R. Geigy AG , which at blowflies a screening of different chemicals carried out on their insecticidal activity. Geigy brought DDT onto the market in 1942 under the trade names Gesarol (agent for crop protection and against stored pests) and Neocid (hygiene area).
War years
In the German Reich there was particular interest in DDT because of its effectiveness against the Colorado beetle . The Reich Ministry of Food and Agriculture placed an order for 10,000 tons of Gesarol in 1942 , which corresponded to around 500 tons of pure substance. Since this exceeded its own capacities, Geigy signed a license agreement with Schering AG in 1943 . In April 1943, the Geigy branch in Grenzach , Baden , began to manufacture DDT. At the end of 1942 the Wehrmacht introduced the Lauseto preparation from IG Farben to control lice . At the beginning of 1944 an analysis in the Schering laboratories showed that it contained 15% DDT. IG Farben then assured Geigy that it would pay license fees for Lauseto and its newly developed fly agent Gix . The production volume of DDT in the German Reich was never exhausted, because IG Farben did not supply the chloral required for production in the required quantities. Other products, e.g. B. on the basis of hydrocyanic acid (such as the well-known Zyklon B ) used in pest control.
In the United States of America, even before 1941, advances in medicine and health care had largely kept all life-threatening infectious diseases under control. After the entry into World War II, US soldiers also had to be protected from common diseases such as malaria and typhus in the war zones. Here, the use of DDT should prove to be a straightforward solution to a number of complex problems.
The American Department of Agriculture received some Gesarol samples from Geigy around the middle of 1942 . During the years 1942 and 1943, DDT was extensively studied in the USA for its effectiveness and possible harmful effects. From mid-1944, methods for efficient production and application were increasingly developed. At the end of 1944, around 900 tons of DDT were being produced per month for the US military, and at the end of the war it was around 1,350 tons per month.
One of the first major deployments of DDT was for lice control in a typhus - epidemic , which in 1943/44 Naples had broken out. Systematic and consistent control of the disease-transmitting lice with pyrethrum and DDT made it possible to contain the disease. With the help of a new procedure at the time, large parts of the population could be deloused quickly; the insect powder was blown between the layers of clothing with a powder atomizer on the collar. The press attributed the success mainly to DDT, henceforth it was considered a “miracle cure” against insect-borne diseases.
Most of the DDT has been used to prevent malaria in the South Pacific. Whole islands were sprayed with DDT solution from the aircraft; the application rate was low at 220 to 280 g DDT / ha . The equipment of the American soldiers deployed in Southeast Asia included a spray can with DDT or pyrethrum to make makeshift shelters mosquito-free. Towards the end of the war, DDT had become the standard means of disinfestation for soldiers, prisoners of war, and the civilian population in the US military .
Agriculture
The War Production Board of the USA approved DDT for civil use on August 1, 1945. With press reports on the successful fight against malaria and typhus during the war, expectations were high. The FDA had concerns about a quick release because DDT had shown liver damage in rats when examined. There was already evidence that it could build up in the body and in milk. Since the FDA did not have the authority to prevent a release at the time, it set a provisional maximum level of 7 ppm for food. No DDT at all should be tolerated in milk. The price per pound of DDT was initially more than $ 1 but dropped to $ 0.25 by the mid-1950s.
In the period that followed, DDT was used in many places as a pesticide in agriculture . In fruit and wine growing , DDT soon replaced the lead arsenate that had been widely used until then . It was also used in household bug sprays.
In 1962, DDT was registered for 334 different agricultural uses in the United States alone. DDT was the most widely used insecticide in the world for several decades. The application rates in agriculture ranged from 0.5 to 35 kg DDT / ha, depending on the crop. The use of DDT was particularly high in cotton cultivation .
Major actions and forestry
In 1950, DDT and HCH were tested in Switzerland in the so-called "cockchafer war". The insecticides were applied along the edges of the forest with motorized sprayers , fog blowers and spray planes . Numerous other insects also fell victim to these actions, beekeepers had to be compensated, and there were protests among nature conservationists and the population. The accompanying scientific evaluation showed that forest edges treated with DDT were avoided by the cockchafer , which simply switched to untreated stands. Geigy presented the actions as a success and was able to enforce the continuation of the cockchafer control with the help of politics.
From 1947 to the 1960s, DDT was used in the USA against the elm splint beetle , the vector of the fungus responsible for Dutch elm disease . The elm was a common avenue tree in the suburbs, where DDT was also sprayed. Because of the high dosage (around 700 g DDT / tree), there was numerous acute poisoning in birds. In some places where many elms had been treated with the insecticide, the songbirds disappeared completely. Conservationists and scientists became aware of the environmental effects of DDT and began to deal with it.
To combat the gypsy moth , around 12,000 km 2 , mostly in New York State , were sprayed with DDT from an airplane in 1956 . There were also suburbs and farmland on the treated area. Since DDT had ended up on pastures, the milk from the cows from these pastures was no longer for sale. There were also fish deaths. Some Long Island residents tried to legally stop the spraying program ( Long Island case ) but were unsuccessful.
In the GDR, DDT was used against the bark beetle . Because of the heavy infestation of the forests, a total of around 600 tons of DDT were applied there in 1983/84, which, however, was an unusually large amount.
Fight against malaria

When using DDT to combat malaria, the interior walls of houses and huts are sprayed with a DDT suspension ( Indoor Residual Spraying , IRS). When the malaria mosquitoes ( Anopheles ) settle there, they ingest a lethal dose of DDT. Since DDT remains effective on the wall for about six months, the spraying action must be carried out twice a year, in the case of seasonal malaria only once. 1–2 g of DDT are required per square meter of wall surface.
From the mid-1950s, the WHO started the Global Eradication of Malaria Program . Re-infection as a result of mosquito bites should be prevented by spraying the walls with DDT solution. At the same time, those who are already ill should be treated with medication. The campaign was initially extremely successful. In India , the number of new malaria infections per year was reduced from 100 million (1952) to 50,000 (1961). Similar successes were also achieved in Pakistan , Ceylon (now Sri Lanka ), Paraguay, Venezuela, Mexico and Central America. Malaria was eradicated in Europe by the late 1960s.
For various reasons, the number of malaria infections increased again in some of the tropical countries involved. Since in the meantime different species of anopheles mosquito had developed resistance to DDT, the resumption of the DDT spray programs did not bring the same success as with the first use. In 1972 the WHO had to admit that the ambitious goal of eradicating malaria worldwide could not be achieved. In the period that followed, damage limitation through malaria control was the official goal.
In the fight against malaria, the use of DDT and other organochlorine pesticides remained standard even after the eradication program ended in 1972. From 1992 the WHO recommended mosquito nets impregnated with pyrethroids . From 1993 onwards, major projects based on the use of DDT were not considered " sustainable ". According to a recommendation by the WHO in 1997, DDT should only be used as part of "integrated" programs. In 2006, the WHO expressly recommended the use of DDT inside buildings, as the expected effect on the environment was small, while the vectors (disease-transmitting insects) that settle on the walls before and after the blood meals could be easily reached .
Prohibition in the USA
The damaging effects of DDT on birds became known in the mid-1950s . In 1962, the American biologist Rachel Carson published the book Silent Spring (" The Silent Spring "), with which she made the problems and risks of pesticide use known to a broad public. The book sparked a sometimes heated debate in the USA about the use of DDT. The large-scale use (such as against the gypsy moth) and the use of very high doses (such as against the elm bark beetle) were soon considered abusive even among DDT proponents and were discontinued.
The bird protection organization Audubon Society set up the Rachel Carson Fund in 1965 to take legal action against the use of DDT. The Environmental Defense Fund , launched in 1967, followed the same tactic. They had a breakthrough in 1969 at a public hearing in the state of Wisconsin . The question was whether the use of DDT is safe for humans and animals. The representatives of the USDA had to admit in cross-examination that they had not carried out any toxicity tests of their own but that they had adopted the manufacturer's information. The final report of the hearing recommended that the use of DDT in Wisconsin be discontinued. In the meantime, President Nixon had set up an advisory body that, in November 1969, recommended phasing out DDT. Nixon decided that US government agencies should stop using DDT after a transition period of two years.
The head of a hearing by the Environmental Protection Agency , Edmund M. Sweeney, came to the conclusion in his final report that there was no violation of the law due to a lack of warnings, that DDT does not pose a disproportionate risk in comparison to the benefits when used properly and that substitutes for DDT are sometimes significantly more dangerous be. EPO Administrator William D. Ruckelshaus did not follow this recommendation and on June 14, 1972 issued a ban on the use of DDT in agriculture, which was to come into force after six months. The reasons given include its longevity, biomagnification and toxicological effects as well as the availability of effective and ecologically less harmful substitutes. The use for disease control as well as the export remained allowed.
Some DDT manufacturers and users tried to appeal this decision; however, their action was dismissed in December 1973 by the Washington Court of Appeals . For its part, the Environmental Defense Fund tried unsuccessfully to impose a ban on the manufacture and export of DDT. In 1973 and 1974, the EPA issued exemptions for use against the striped scarab beetle (pea leaf weevil). In 1974 a large-scale DDT use against a carrier spinner species ( English Douglas fir tussock moth ) in the forests in the northwest of the USA was approved.
A few days before the end of his term in 1981, President Carter issued Executive Order 12264, which banned the export of chemicals not permitted in the USA, including DDT. However, his successor Reagan quickly repealed this decree. The last remaining manufacturer in the USA, Montrose Chemical, ceased DDT production for economic reasons in June 1982.
Bans in Europe
Silent Spring was also successful in Europe, but the media and public discussion remained less. The DDT issue was nowhere near as important to politics and environmental movements as it was in the United States. Discussions and decisions about the approval or prohibition of DDT took place in the responsible specialist committees. The development in the USA was closely followed there.
In the spring of 1968, the USA and Canada banned the import of Swiss cheese because it exceeded the maximum levels of lindane , dieldrin and DDT. The main cause was identified as being an insecticide-containing paint that had been used to paint many cow stalls to control flies.
Sweden became the first European country to ban the use of DDT with effect from January 1, 1970. In the spring of 1970, the Federal Research Institute in Wädenswil restricted the use of DDT to eleven insect species. A discussion in politics and the media only got underway a year later, in spring 1971. In Switzerland from January 1972, the use in agriculture was no longer permitted. When the Federal Poison Act came into force on April 1, 1972, all other uses of DDT were also banned there. The government of the Federal Republic of Germany announced in the summer of 1971 that it intended to ban the use of DDT. As a result, an application ban was worked out with the DDT Act and passed in August 1972. The manufacture and sale of DDT have been prohibited in the Federal Republic of Germany since July 1, 1977. In the years that followed, DDT was rarely used in Austria, but it was not banned until 1992.
Further development and today's situation
In numerous developing countries, but also in the states of the Eastern Bloc , DDT was still produced and used. DDT was originally much more important in agriculture and forestry in the GDR than in West Germany. However, the use of DDT also fell sharply here in the course of the 1970s. In the end it was only used for pickling onion seeds.
DDT was contained in the wood preservative Hylotox 59 , which was produced in the GDR until 1988. Therefore, DDT can still often be detected in buildings in eastern Germany . It was allowed to be used temporarily until June 30, 1991. Today, this wood preservative is generally considered a building pollutant in buildings .
In India, the use of DDT in agriculture was banned in May 1989. It is still used there today to fight malaria. As part of the five-year plan, which runs until 2007, 66,000 t of DDT powder (active ingredient content 50%) should be used to combat malaria and leishmaniasis . The effectiveness against malaria vectors is currently the subject of controversial discussion among Indian scientists.
The Stockholm Convention of May 2001, which came into force in May 2004, limits the use of DDT to the control of disease-transmitting insects ( vectors ). In addition, it can continue to be manufactured as a raw material for the production of the acaricide dicofol . The use of DDT should be communicated to WHO and the Secretariat of the Stockholm Convention. There is evidence that at least 21 states are using DDT. The registered states are to report every three years on the amount of DDT used, its use and the disease control strategy.
In September 2006 the director of the “Global Malaria Program” of the WHO announced that the use of DDT should be increased again in the future. As a result, DDT consumption was expected to increase somewhat in the following years, but this did not occur. In October 2008, 15 states reported the use of DDT for disease control.
There have been occasional reports of continued use of DDT in agriculture, such as in India, North Korea and possibly other countries.
India plans to end the use of DDT by 2020.
Production quantities
The production figures for DDT have not been consistently collected and published in all countries. The USA was the main producer of DDT for a long time, with 74,600 t in 1960 and 26,900 in 1970. Only the production data for 1965 are known from the Federal Republic, at that time it was the second largest DDT manufacturer in the world with 30,000 t. In the second half of the 1960s, between 15,000 and 25,000 tonnes per year were produced in the USSR ; in Italy it was 10,000 tonnes per year. In 1981, around 9,500 t were still produced in the EU countries .
For 2005 the world annual production of DDT was estimated at 6,269 t of active ingredient, which was split between India (4,250 t) and China . It is believed that around 300 t were also produced in North Korea . The production and use of DDT in China ceased in 2010. The only known manufacturer that remains is the Indian Hindustan Insecticide Ltd., which produced 3,872 t of DDT in its 2012/2013 financial years and 2,786 t of DDT in 2013/2014. Of these, 287 t and 75 t respectively were exported.
Manufacturing
In the classic manufacturing process for DDT, chloral and chlorobenzene reacted in concentrated sulfuric acid to form DDT. If some of the sulfuric acid was replaced by fuming sulfuric acid , chloral hydrate could also be used. The sulfuric acid absorbed the water formed during the reaction. The reaction product was washed and melted in boiling water with the addition of a little lye in order to remove adhering acid residues. When the solution cooled, DDT precipitated out in solid form. The melting point of the technical mixture was around 90 ° C. DDT could be obtained in its pure form by recrystallization in ethanol or propanol.
In the USA, an alternative manufacturing process was developed in the mid-1940s that did not require large amounts of sulfuric acid. The starting materials were chloral hydrate and chlorobenzene; instead of sulfuric acid, chlorosulfonic acid was used. The chloral hydrate formed alkyl sulfates with chlorosulfonic acid as an intermediate . The sulfate residues were replaced by chlorobenzene in the next reaction step. Towards the end of the process, carbon tetrachloride was added as an inert solvent to prevent the reaction product from clumping. The solvent was separated from the washed and neutralized product by steam distillation. The yield in this process was 77%, while it could reach over 90% with chloral and sulfuric acid.
Isomers and metabolites
Technical DDT is an amorphous white powder, its melting point is between 80 and 94 ° C.
In technical DDT, different isomers and by-products could be detected in different concentrations:
| p , p ′ -DDT | o , p ′ -DDT | p , p ′ -DDD | o , p ′ -DDD | p , p ′ -DDE | o , p ′ -DDE | other | reference |
|---|---|---|---|---|---|---|---|
| 77.1 | 14.9 | 0.3 | 0.1 | 4th | 0.1 | 3.5 | IPCS 1989 |
| 65-80 | 15-21 | ≤ 4 | ≤ 1.5 DDOH | UBA 1993 |
The p , p 'isomers are often called 4,4'-isomers, the o , p ' isomers 2,4'-isomers.
The main component of technical DDT and essentially responsible for the insecticidal effect is p , p ′ -DDT or 1,1,1-trichloro-2,2-bis ( p -chlorophenyl) -ethane (CAS No. 50-29-3 ). In practice, p , p ′ -DDT is not used in its pure form, but the technical mixture.
o , p ′ -DDT (CAS No. 789-02-6) is the most common contamination in technical DDT with proportions of 15 to 21%. It contributes only marginally to the insecticidal effect, but has a relatively strong estrogenic effect.
D ichlor d iphenyldichloreth e n, 1,1-dichloro-2,2-bis ( p -chlorophenyl) ethene or p , p '-DDE (CAS no. 72-55-9) is in the technical mixture with about 4% contain. In the human body, p , p ′ -DDT is mainly broken down into p , p ′ -DDE. p , p ′ -DDE was mainly responsible for egg shell thinning in birds of prey.
2,4-DDE (CAS No. 3424-82-6) only accounts for 0.1% of technical DDT. It is created by the breakdown of o , p ′ -DDT.
D ichlor d iphenyl d ichlorethan , 1,1-dichloro-2,2-bis ( p -chlorophenyl) ethane or p , p '-DDD (CAS no. 72-54-8) was prepared by condensation manufactured by dichloroacetaldehyde with chlorobenzene and used as an insecticide. Production figures are not known, it was of little importance. In the 1950s, DDD was used to control mosquito larvae in the waters of Clear Lake . Through biomagnification, it accumulated in the food chain and led to the collapse of theracing divers population at this lake.
o , p '-DDD or 1-chloro-4- [2,2-dichloro-1- (2-chlorophenyl) ethyl] benzene (CAS No. 53-19-0) is in technical DDT with a proportion of about 0.1% included. It is used in veterinary medicine under the active ingredient name Mitotane to treat Cushing's syndrome in dogs , but is becoming increasingly less important than more modern drugs due to its toxic properties.
The two benzene rings in the DDT molecule are not coplanar but twisted against each other.
Analytics
From 1945 a variant of the Schechter-Haller method was used to determine the DDT content. In the process, DDT was extracted from the samples and nitrated to polynitro derivatives, which formed a dye after addition of a methylating agent . The content of the dye could be determined on the photometer and thus the DDT content of the sample could be calculated. The method was improved in 1953 so that some of the DDT derivatives could also be quantified with it.
From around 1962, gas chromatography was a very good separation process for DDT and its breakdown products. Initially, the electrochemical detector or the electron capture detector were mostly used as detectors . A mass spectrometer coupled to the gas chromatograph later became the preferred detector.
Mode of action
DDT acts primarily on the central nervous system . With low doses it comes to over-excitability, with high doses to paralysis . The increase in excitability occurs first in the motor neurons of the brain, spinal nerves are only affected at higher concentrations. When exposed to DDT, nerve cells are stimulated to “fire” spontaneously, causing muscles to contract. This leads to tremors of the body and limbs, the so-called "DDT jitters ". DDT leads to an increased release of neurotransmitters to small postsynaptic potentials on the motor end plates , the transitions between the nervous system and the muscles. This “consumes” neurotransmitters, which ultimately makes stimulus conduction impossible. Over the course of a few hours or days, DDT paralyzes and eventually kills the insect. Compared to other insecticides, the effect of DDT occurs rather slowly, although it is more effective at low temperatures than at high temperatures.
In the membrane of the nerve cells of insects there are “Para” - sodium channels , the name of which goes back to their location in the so-called paralysis area on the X chromosome of Drosophila . They are voltage-controlled and enable sodium ions to flow in during depolarization , i.e. when a nerve impulse is triggered. During the subsequent repolarization , the rebuilding of the resting voltage, and in the resting state, the sodium channels must be closed. DDT can attach to the sodium channels and prevent them from closing. The attachment point is probably an elongated hydrophobic cavity, the DDT molecule only extends into its upper part. The acid group of the faster pyrethroids , which work in an even lower dose , accumulates in the same area as DDT, but their alcohol group extends deeper into the cavity.
resistance
Serious problems with DDT resistance first appeared in stable flies in northern Sweden in 1946. As a countermeasure, Geigy AG increased the active ingredient content of its Gesarol spray from 5 to initially 10%, and a few years later to 50%. When resistant flies appeared in many places in Switzerland in 1949, DDT sales fell noticeably and Geigy accelerated the development of the Diazinon, which was intended to be the successor to DDT .
As early as 1953, the WHO was aware of cases of DDT resistance in malaria-transmitting Anopheles mosquitoes. To reduce the time it takes for resistance to develop, DDT should only be used for a few years during the “attack phase ” of the Global Eradication of Malaria Program . Nonetheless, resistant Anopheles mosquitoes appeared in El Salvador, Mexico and parts of India, among others, and DDT use in agriculture is also to blame.
The most common form of resistance, the "knockdown resistance" (kdr), leads to an approximately 14-fold higher tolerance for DDT, pyrethrins and pyrethroids. It goes back to a mutation in the para sodium channel that has now been found in many insect species. The “super kdr” resistance, which is important for pyrethroids, is of little importance for DDT.
The use of DDT and pyrethroids in agriculture led to the emergence of resistant mosquitoes even where DDT was never used to combat malaria. According to a UNEP report from 2007, 64% of the populations of the most important malaria vector Anopheles gambiae in Africa showed resistance to DDT in random samples , around a third of which were highly resistant. DDT resistance is also widespread in Ethiopia and India.
toxicology
The acute toxicity of DDT for humans and mammals is low compared to other organochlorine pesticides. The highest DDT dose reported in the literature in humans was 285 mg / kg body weight and was survived. The LD 50 in rats (oral) is around 250-300 mg / kg body weight. Acute poisoning manifests itself primarily in neurotoxic (nervous) effects such as tongue numbness, dizziness, twitching of the facial muscles through to seizures and paralysis.
The biological half-life , i.e. the period of time that the body needs until half of the ingested DDT has been broken down or excreted, is more than a year in humans. In humans, p , p ′ -DDT is mainly broken down into p , p ′ -DDE. o , p ′ -DDT is eliminated faster than p , p ′ -DDT.
In humans, a possible connection between DDT exposure and reduced sperm counts could not be clearly proven.
The association between DDT exposure and different types of cancer in humans has been investigated in countless studies. The carcinogenic effect of technical DDT, p , p ′ -DDT and p , p ′ -DDE has been proven beyond doubt on rodents . It is still unclear to what extent these results can be transferred to humans. In long-term studies in rats, mice and hamsters, the tumors formed in the liver, lungs and the lymphatic system , but not in the breast or genital organs. The carcinogenic effect is possibly due to the hormonal effectiveness. The International Agency for Research on Cancer (IARC) of the WHO classified DDT in 2015 as “probably carcinogenic in humans” (group 2A).
A genotoxic effect in humans could not be clearly demonstrated. In some studies, chromosomal aberrations were found in occupationally exposed persons . However, they were also exposed to other pesticides and it is unclear whether other risk factors have been adequately addressed. Laboratory tests to establish a genotoxic effect produced contradicting results.
A study was able to show a connection between the occurrence of premature labor in 20 Indian women and, compared to the control group, increased concentrations of p , p ′ -DDE and p , p ′ -DDT in blood and placental tissue. However, the levels of hexachlorobenzene , lindane and aldrin were also increased in the women with premature labor. Other studies provided evidence of a connection between increased concentrations of p , p ′ -DDT and the occurrence of stillbirths or between p , p ′ -DDE exposure and a shortened breastfeeding period . It was not until 2014 that a study showed that DDE may be involved in the development of Alzheimer's. Compared to a control group, patients with Alzheimer's had a 3.8-fold higher DDE value in the serum.
Endocrine effect
DDT and some of its breakdown products can act as endocrine disruptors , i.e. act similar to hormones in living beings or inhibit natural hormones.
DDT acts as an agonist on the estrogen receptor , where it is deposited and acts like estrogen. The strongest estrogenic effect has o , p ′ -DDT, especially the levorotatory enantiomer , followed by o , p ′ -DDE. The p , p ′ isomers of DDT and DDE have almost no estrogenic effect.
At the androgen receptor , DDT and its breakdown products prevent the accumulation of endogenous androgens, but do not have an androgenic effect themselves. This effect as androgen antagonist is at p , p '-DDE more pronounced than in p , p ' -DDT and o , p '-DDT. Natural hormones bind much more strongly (factor 10 3 to 10 6 ) to estrogen and androgen receptors than DDT or DDE.
The endocrine effect of DDT and its derivatives is now considered to be the cause of various types of reproductive disorders that occurred in living beings from different classes of animals. The best known of these is egg shell thinning in birds.
Exposure of humans
In the early years, no special occupational safety measures appeared to be necessary when handling the end product. Very high levels of DDT were found in the blood and body tissue of workers in DDT production. Since no harmful effects were observed, this was taken as an indication of the harmlessness of DDT.
In western countries today, DDT is mainly ingested through foods of animal origin. Until a few years ago, residues of pesticides in imported food were also a possible source.
In Germany it was found in investigations in the late 1990s that p , p ′ -DDT and its breakdown product p , p ′ -DDE occurred in a ratio of about 1: 9 in blood serum . At the beginning of the 1970s, the proportion of DDT in serum was higher, the ratio was up to 3: 1. A higher proportion of DDT in the blood serum compared to DDE indicates a recent intake, as it can still occur in third world countries. The o , p ′ isomers are broken down more quickly in the body and make up only 1% of the total DDT in blood serum.
At the beginning of the 1990s, the mean p , p ′ -DDE content of the serum in the age group from 21–30 years was 1.5 µg / l, whereas in the age group from 51–60 years it was found to be 3.3 µg / l (old federal states).
The mean total DDT exposure of breast milk in (West) Germany fell between 1980 and 1994 from around 1910 µg / kg fat to 367 µg / kg fat. In the new federal states, however, in 1990 it was still around 2250 µg / kg fat. In the USA in 1955 an average of 15 mg DDT / kg adipose tissue was found , by 1980 this value had fallen to 5 mg / kg. By the late 1980s, adipose tissue concentrations had dropped to around 1 mg / kg in the United States, Canada, and Europe.
In infants , the total DDT levels do not differ from those of adults. Children take in DDT isomers through the placenta and later through their breast milk.
In countries where DDT was used until recently or is still used today, the DDT levels in blood, breast milk and adipose tissue are significantly higher. The exposure was particularly high among workers in DDT production. In the mid-1960s, total DDT levels between 38 and 647 mg / kg adipose tissue and around 350 to 740 µg / kg serum were found in them.
Environmental behavior and ecotoxicology
Environmental behavior
DDT is only slowly broken down in nature, and its breakdown usually begins with the conversion into the likewise very long-lived compounds DDE and DDD.
In the soil , DDT, DDD and DDE adsorb strongly on organic soil components and clay minerals . As a result, they hardly ever get into the groundwater, but in the event of heavy rainfall they can be carried into water with washed away soil. Over the years, they also diffuse into the micropores of the soil, where they are not available for microbial degradation. DDT and its conversion products can be broken down by a wide range of bacteria and fungi. If oxygen is available, the first step is mainly DDE; under reducing conditions, the breakdown to DDD predominates. The rate of degradation depends on the activity of the soil life ; it increases with higher temperatures and good nutrient and water supply. DDT and its conversion products can evaporate from the soil into the atmosphere, which is favored by high temperatures and flooding of the soil. When determining the half-life of DDT in soils, all discharge routes used to be recorded as "degradation". In some cases, only the insecticidally effective p , p ′ -DDT was considered, without taking into account the high persistence of the degradation products. In the tropics, DDT “disappears” from the soil faster than in cooler climates. In a study carried out in the 1980s, the half-life - based on total DDT - in tropical and subtropical countries was 22 to 365 days. In comparison, half-lives of 837 to 6087 days (16.7 years) were found in temperate climatic zones.
In the atmosphere, half of DDT is in the gas phase and half is in particle-bound form . The DDT in the gas phase is mainly broken down by hydroxyl radicals with a half-life of about 37 hours. Particle-bound DDT is not subject to this degradation and can be transported over great distances in the atmosphere. Most of the atmospheric DDT is believed to be washed out by precipitation.
On the surface of water, DDE can be decomposed by photolysis within a few days, DDT and DDD are only broken down very slowly in this way. Biodegradation hardly takes place in free water. DDT is broken down into DDE by hydrolysis ; this reaction is favored by a basic environment.
Due to the lipophilic properties of DDT, DDE and DDD (log K OW : 6.36, 5.70 and 5.50), these accumulate via the food chain in the fatty tissue of humans and animals ( bioaccumulation ). Bioconcentration factors of 12,000 ( rainbow trout ) to 100,000 are given for fish, 4,550 to 690,000 for mussels and 36,000 for snails . Fish ingest DDT both directly from the water and with their food. Migrating schools of fish can carry DDT from heavily polluted waters to regions that were originally less polluted.
DDT is one of the compounds that accumulates on the surface of plastic debris floating in the ocean .
Birds
DDT and its metabolite DDE accumulate strongly in the food chain; the highest DDE contamination was therefore found in bird- and fish-eating birds of prey. In some bird species, DDE leads to egg shell thinning. In animal experiments, chickens and quails proved to be insensitive to the eggshell thinning caused by DDT metabolites. Ducks and pigeons were moderate, but many birds of prey were very sensitive.
In birds , o , p ′ -DDT is rapidly metabolized and excreted, while p , p ′ -DDT is only slowly broken down to DDE.
A large-scale catastrophic decline in the peregrine falcon population was discovered in the UK in 1961 . A census in 1962 found a population decline of 44% for the whole country compared to the mean population for 1930–39. In the south of England the species had completely disappeared, in Wales and in the north of England the population had declined sharply and only in the Scottish Highlands the population decline was relatively small. Irrespective of this, from 1951 onwards, heaps of broken eggs were found in peregrine falcon nests, which was previously practically unknown. After the collapse of the population was discovered, older peregrine falcon egg shells from egg collections in museums and from collectors were examined and a sudden decrease in egg shell thickness by an average of around 20% from 1947 onwards was found. Similar reductions in egg shell thickness have also been found in the UK in sparrowhawk and merlin .
Catastrophic populations and a significant decline in egg shell thickness after 1950 were recorded simultaneously or only a little later in large parts of the northern hemisphere. In Europe, the peregrine falcon died out in Denmark, the Netherlands, Belgium, Luxembourg and the GDR by the end of the 1970s, the populations in Scandinavia , the then FRG, Switzerland, Austria and Poland declined except for a few pairs. The tree breeder population in Central and Eastern Europe died out completely. In the United States, the peregrine falcon disappeared from all states east of the Rocky Mountains .
The sudden decrease in egg shell thickness after 1946 occurred at the time when DDT was first widely used in agriculture and forestry. At the end of the 1960s it was found that the content of the DDT metabolite DDE in eggs correlated negatively with the thickness of the egg shell . A 17% decrease in egg shell thickness was associated with a DDE content of 15-20 ppm DDE based on the fresh weight of the egg content. Peregrine falcon populations, whose average eggshell thicknesses were reduced by 17% or more, fell sharply or died out.
As early as 1958 it was reported that bald eagles were rarely raising young in the USA. Similar effects occurred in cormorants on the Great Lakes in Canada in the early 1970s . Here the population had decreased to 100 breeding pairs. The egg shell thickness was reduced by more than 20% compared to eggs collected before 1945. The average DDE content of the cormorant eggs in 1972 was 22.4 mg / kg fresh weight. Problems with rearing young could also be traced back to DDT or its breakdown products in the case of sparrows in the British Isles, bald eagles on the Great Lakes and ospreys in southern Sweden. The birds were also exposed to other environmental pollutants such as PCB , mercury , dioxins , chlordane and dieldrin at that time. In the statistical analysis of the results, the respective DDE exposure always gave the best explanation for the eggshell thickness or the lack of breeding success.
The mechanism by which the decrease in eggshell thickness comes about has not yet been clarified beyond any doubt. For example, a disruption of calcium storage in the egg shell via an inhibition of calcium ATPase and carbonic anhydrase is being discussed . The synthesis of the hormone prostaglandin , which is also responsible for the hydrogen carbonate transport, is also influenced. There are indications that DDE, similar to progesterone (progesterone-mimetic), inhibits ovulation and causes an increase in the avidin content in the fallopian tube .
In southern California in the 1950s and 1960s, DDT-containing wastewater from a factory ended up in the sea. In the western gulls ( L. occidentalis ) living there , the sex ratio was shifted towards the females. The proportion of female-female pairs was significantly increased with 10% of the breeding pairs. There were an unusually large number of eggs in the nests, but some of them had not been fertilized. For this, one has feminization of male bird embryos by the estrogenic effect of o , p blamed '-DDT.
The total DDT content in tissue from birds decreased between the early 1970s and 1980s in the northern hemisphere, while the relative proportion of the main metabolite DDE increased. Since the early 1990s, DDT levels have remained more or less constant, albeit at a low level.
Mammals
Young gray seals from the North Sea and North Atlantic had 1.2–2.5 mg total DDT / kg fat in the 1980s. For gray seal pups from the Baltic Sea, the total DDT concentrations were around a factor of 20 higher. The number of seals in the Baltic Sea decreased, the newborns had a higher mortality , there were lesions of the skull bone and occlusion of the uterus . All gray seals were exposed to PCBs at the same time, the PCB concentrations were about twice as high as the DDT concentrations.
When Florida Panther (sperm count, sperm abnormalities, decreased in the 1990s could reproductive disorders undescended testicles ) on its high exposure to the antiandrogen effective p , p '-DDE (5-60 mg / kg liver) are returned. The influence of estrogenic substances such as PCBs (7–26 mg / kg liver) and inbreeding could not be excluded. The LD 50 for mammals is in the range of 0.1-0.5 g DDT / kg body weight. In experiments on the long-term effects of DDT, harmful effects occurred in rabbits after daily intake of more than 0.184 mg DDT / kg body weight ( NOAEL ).
Other classes of animals
In reptiles , sex determination can be influenced by external factors, sometimes also by endocrine disrupting substances.
In Lake Apopka in Florida came after a chemical accident in 1980 dicofol, DDT, DDD, DDE and sulfuric acid. Within the next four years, the population of the pike alligator ( Alligator mississippiensis ) decreased by 90%. The mortality of the adult animals was clearly higher, that of the young animals was drastically higher than in a control population. The sex ratio was shifted towards the females and changes in the hormone level and in the sexual organs of the alligators were found. In the laboratory, DDE was used to trigger a gender transition or intersexuality on the eggs of the pike alligator . In snapping turtles ( Chelydra serpentina ) in Canada reduced was found sexual dimorphism , which is probably linked to exposure to p , p stands' -DDE or PCB related.
Foreign substances can also lead to endocrine disorders in amphibians and crustaceans . It is still unclear whether DDT and its derivatives also show such an effect on these animal classes.
Fish can only break down DDT slowly; p , p ′ -DDE and p , p ′ -DDD have been identified as metabolites in them. For some fish (Baltic cod ) a decrease in pollution has been observed since the 1970s, for others (Baltic herring , North Sea dab ) no trend can be seen. In in-vivo studies, DDT and its derivatives caused estrogenic effects in fish; these results could be confirmed in vitro . These effects are very dependent on the respective fish species and their stage of development and are difficult to transfer to other species.
In the case of insects , in addition to acute toxicity, there is also evidence of endocrine effectiveness. In nymphs of the bug Rhodius prolixus , the molting frequency was increased after exposure to DDT, in the adults the time to oviposition was shortened. In vitro , the competitive binding of DDT and juvenile hormone to an adipose body protein was shown in the butterfly Heliothis zea .
Individual evidence
- ↑ a b c Entry on DDT. In: Römpp Online . Georg Thieme Verlag, accessed on April 13, 2014.
- ↑ a b c d e f g h Entry on 4,4´DDT in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ a b c d e f g h U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry: Toxicological profile for DDT, DDE and DDD. (PDF; 4.2 MB) , 2002.
- ↑ Entry on Clofenotane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Lukas Straumann: Useful pests. Chronos Verlag, Zurich, 2005, ISBN 3-0340-0695-0 , pp. 236–245.
- ↑ David Kinkela: DDT and the American century. The University of North Carolina Press, Chapel Hill, 2011, pp. 12-14.
- ↑ a b c d e f g h i Christian Simon : DDT - cultural history of a chemical compound. Christoph Merian Verlag, Basel, 1999, ISBN 3-85616-114-7 .
- ^ Charles M. Wheeler: Control of Typhus in Italy 1943/1944 by Use of DDT. American Journal of Public Health , Feb. 1946, Vol. 36, pp. 119-129; PMC 1626020 (free full text).
- ↑ a b c d e f g h i Thomas R. Dunlap: DDT: Scientists, Citizens and Public Policy. Princeton University Press, 1981, ISBN 0-691-04680-8 .
- ↑ a b c d e f g h i j k l m n o Advisory committee for existing substances of the Society of German Chemists : DDT and derivatives - model substances to describe endocrine effects relevant to reproduction. BUA-Stoffbericht 216, S. Hirzel Verlag, August 1998, ISBN 3-7776-0961-7 .
- ↑ Lukas Straumann: Useful pests. Chronos Verlag, Zurich, 2005, ISBN 3-0340-0695-0 , pp. 288-296.
- ↑ WHO: WHO gives indoor use of DDT a clean bill of health for controlling malaria. Press release from September 15, 2006.
- ↑ Sweeney, Edmund M, Consolidated DDT hearing: hearing examiner's recommended findings, conclusions, and orders, Environmental Protection Agency, April 25, 1972; (Excerpts) (full document, 56 MB) ( Memento of May 11, 2012 in the Internet Archive ).
- ↑ Environmental Protection Agency: Consolidated DDT Hearings: Opinion and Order of Administrator. June 30, 1972 (PDF, 1.7 MB) .
- ↑ United States Environmental Protection Agency website: DDT Regulatory History: A Brief Survey (to 1975).
- ↑ David Kinkela: DDT and the American century , The University of North Carolina Press, Chapel Hill, 2011, pp. 179-180.
- ↑ a b c Werner Perkow: "Active substances in pesticides and pesticides". 2nd edition, Paul Parey publishing house.
- ↑ Greenpeace Austria: Chlorine pesticides and PCBs ( Memento of November 16, 2012 in the Internet Archive ) (PDF; 24 kB).
- ↑ Information sheet Hylotox 59 - DDT and lindane in interiors. In: www.lagus.mv-regierung.de. State Office for Health and Social Affairs Mecklenburg-Western Pomerania, accessed on May 16, 2016 .
- ↑ Structural and technical systems contaminated with pollutants - demolition, renovation and maintenance work . In: Association of German Engineers VDI / General Association of Pollutant Restoration (Ed.): VDI guidelines . VDI / GVSS 6202 Part 1, October 2013.
- ↑ a b V.P. Sharma: DDT: The fallen angel. (PDF; 74 kB). 2003, Current Science, 85, 11, 1532-1537.
- ↑ K. Gunasekaran, SS Sahu, P. Jambulingam, PK Das: DDT indoor residual spray, still an effective tool to control Anopheles fluviatilis-transmitted Plasmodium falciparum malaria in India. 2005, Tropical Medicine & International Health 10 (2), 160-168; doi : 10.1111 / j.1365-3156.2004.01369.x .
- ↑ POPs Convention (2004) ( Memento of October 26, 2012 in the Internet Archive ) (PDF; 220 kB).
- ↑ a b c d United Nations Environment Program: Report of the expert group on the assessment of the production and use of DDT and its alternatives for disease vector control. ( Memento from May 14, 2012 in the Internet Archive ) (PDF; 85 kB) Third Meeting, Dakar, April 30 to May 4, 2007.
- ^ Arata Kochi: "Help save African babies as you are helping to save the environment." September 15, 2006.
- ↑ a b United Nations Environment Program: Report of the DDT expert group on the assessment of the production and use of DDT and its alternatives for disease vector control. November 12, 2014.
- ↑ Provisional DDT register pursuant to paragraph 1 of part II of annex B of the Stockholm Convention online ( Memento of August 24, 2005 in the Internet Archive ).
- ↑ Concern over excessive DDT use in Jiribam fields . May 5, 2008. Archived from the original on December 6, 2008.
- ↑ Henk van den Berg, Secretariat of the Stockholm Convention : Global status of DDT and its alternatives for use in vector control to prevent disease (PDF; 288 kB) Stockholm Convention / United Nations Environment Program . October 23, 2008. Archived from the original on November 18, 2010. Retrieved November 22, 2008.
- ↑ Deccan Herald : India-United Nations pact to end DDT use by 2020 , August 26, 2015.
- ^ SF Darling: The Laboratory Preparation of DDT. J. Chem. Education , 22, p. 170, 1945, doi : 10.1021 / ed022p170 .
- ↑ Walter HC Rueggberg, David J. Torrans: Production of DDT ... Condensing Action of Chlorosulfonic Acid on Chloral Hydrate and Chlorobenzene. Industrial and Engineering Chemistry, pp. 211-214, February 1946, doi : 10.1021 / ie50434a026 .
- ↑ Environmental Health Criteria (EHC) for DDT and its derivatives , accessed December 9, 2014.
- ^ Federal Environment Agency: Updated update of Substance Report 56 (DDT). Ufoplan 103 40 113, September 1993.
- ^ TP DeLacy, CHL Kennard: Insecticides. Part II. Crystal structures of 1,1-bis- (p-chlorophenyl) -2,2,2-trichloroethane (p, p′-DDT) and 1- (o-chlorophenyl) -1- (p-chlorophenyl) - 2,2,2-trichloroethane (o, p′-DDT). In: Journal of the Chemical Society, Perkin Transactions 2. 1972, pp. 2148-2153, doi: 10.1039 / P29720002148 .
- ↑ WHO (INCHEM): DDT and its Derivatives. Environmental Health Criteria 9, Geneva 1979, ISBN 92-4-154069-9 .
- ^ DG Hayward, JW Wong, HY Park: Determinations for Pesticides on Black, Green, Oolong, and White Teas by Gas Chromatography Triple-Quadrupole Mass Spectrometry. In: Journal of Agricultural and Food Chemistry . Volume 63, number 37, 2015, pp. 8116-8124, doi : 10.1021 / acs.jafc.5b02860 , PMID 26209005 .
- ^ Forth, Henschler, Rummel: General and special pharmacology and toxicology. BI-Wiss.-Verl., 1992, ISBN 3411150262 .
- ↑ a b T.GE Davies, LM Field, PNR Usherwood, MS Williamson: DDT, Pyrethrins, Pyrethroids and Insect Sodium Channels. IUBMB Life 59 (3), March 2007, pp. 151-162.
- ↑ Lukas Straumann: Useful pests. Chronos Verlag, Zurich, 2005, ISBN 3-0340-0695-0 , pp. 261-263.
- ↑ G. Chapin, R. Wasserstrom: Agricultural production and malaria resurgence in Central America and India. Nature 293, Sep 17, 1981, pp. 181-185.
- ↑ IARC Monographs evaluate DDT, lindane, and 2,4-D , June 23, 2015.
- ↑ Jason R. Richardson, Ananya Roy, Stuart L. Shalat et al .: Elevated Serum Pesticide Levels and Risk for Alzheimer Disease. JAMA Neurol., January 27, 2014. doi : 10.1001 / jamaneurol.2013.6030 .
- ^ Rene P. Schwarzenbach, Philip M. Gschwend, Dieter M. Imboden: Environmental Organic Chemistry. Wiley-Interscience, Hoboken, New Jersey 2003, ISBN 0-471-35750-2 .
- ↑ EL Teuten, JM Saquing u. a .: Transport and release of chemicals from plastics to the environment and to wildlife. In: Philosophical Transactions of the Royal Society B: Biological Sciences. 364, 2009, pp. 2027-2045, doi : 10.1098 / rstb.2008.0284 .
- ↑ z. BB Conrad: On the situation of pesticide pollution in birds of prey and owls in the Federal Republic of Germany. In: Birds of prey and pesticides. Ökologie der Vögel 3, 1981, special issue: pp. 161–167.
- ^ DA Ratcliffe: The status of the Peregrine in Great Britain. Bird Study 10, 1963, pp. 56-90.
- ^ DA Ratcliffe: Decrease in eggshell weight in certain birds of prey. Nature 215, 1967, pp. 208-210.
- ↑ DB Peakall, LF Kiff: DDE contamination in Peregrines and American Kestrels and its effect on reproduction. In: Cade et al. 1988, pp. 337-351.
Web links
- Bavarian consumer information system: DDT - dichlorodiphenyltrichloroethane
- Stockholm Convention : Overview - Dichloro-diphenyl-trichloroethane (DDT )