Hexachlorocyclohexane
Hexachlorocyclohexane , more precisely 1,2,3,4,5,6-hexachlorocyclohexane , is the name of a mixture of different stereoisomeric chemical compounds from the group of halogenated hydrocarbons .
overview
The most common compound is γ-hexachlorocyclohexane , which is used as an insecticide and is better known under the name lindane. The other isomeric chemical compounds are α-hexachlorocyclohexane, β-hexachlorocyclohexane and δ-hexachlorocyclohexane. There is also ε-hexachlorocyclohexane in small quantities. The η, θ and ζ isomers were only synthesized in the laboratory. Because the earlier through the manufacturing process (from benzene , which is chlorinated under UV radiation , whereby five of the eight possible isomers are formed) with existing and with released α-, β-, δ- and ε-isomers are more toxic than Insecticide proved to be less effective and even more difficult to break down than the problematic γ-structure (which is contained in technical hexachlorocyclohexane only 9-18%), later only isomerically pure γ-hexachlorocyclohexane was used as a food and contact poison. Lindane (i.e. γ-hexachlorocyclohexane) was first produced in 1825 by Michael Faraday . During thermal decomposition, phosgene and hydrogen chloride , among other things, are formed . α-Hexachlorocyclohexane is the only 1,2,3,4,5,6-hexachlorocyclohexane to be found in two enantiomeric forms: (+) - α-hexachlorocyclohexane and (-) - α-hexachlorocyclohexane. In the insecticide, α-hexachlorocyclohexane is found as a racemate [1: 1 mixture of (+) - α-HCH and (-) - α-HCH] for production reasons. However, mainly (-) - α-HCH was found in the liver of wild deer, and the rate of degradation of (+) - α-HCH is significantly higher than that of (-) - α-HCH. The degradation takes place enantioselectively.
properties
| 1,2,3,4,5,6-hexachlorocyclohexanes | ||||||||||||
| Surname | (-) - α- Hexachlorocyclohexane | (+) - α-Hexachlorocyclohexane | β- hexachlorocyclohexane | γ- hexachlorocyclohexane ( lindane ) | δ- hexachlorocyclohexane | ε -hexachlorocyclohexane | ζ -hexachlorocyclohexane | η -hexachlorocyclohexane | θ -hexachlorocyclohexane | |||
| Structural formula |  |
 |
 |
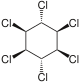 |
 |
 |
 |
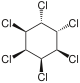 |
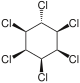
|
|||
| spatial structure (for alpha with hydrogen atoms) |
 |
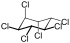 |
 |
 |
 |
|
||||||
| CAS number | 608-73-1 (mixture of isomers) | |||||||||||
| 319-84-6 (racemate) | 319-85-7 | 58-89-9 | 319-86-8 | 6108-10-7 | 6108-11-8 | 6108-12-9 | 6108-13-0 | |||||
| 119911-70-5 | 119911-69-2 | |||||||||||
| PubChem | 727 | |||||||||||
| Molecular formula | C 6 H 6 Cl 6 | |||||||||||
| Molar mass | 290.83 g mol −1 | |||||||||||
| Physical state | firmly | |||||||||||
| Brief description | colorless to yellowish crystalline solid | |||||||||||
| Melting point | 158 ° C | 309 ° C | 113 ° C | 139 ° C | 219 ° C | |||||||
| boiling point | 288 ° C | 323 ° C (decomposition) | ||||||||||
| density | 1.89 g cm −3 | 1.85 g cm −3 | 1.55 g cm −3 | |||||||||
| solubility | very sparingly soluble in water (0.2–8 mg / l) | |||||||||||
| GHS pictograms |
danger |
|||||||||||
| H and P phrases | H: 301-312-351-410 | |||||||||||
| EUH: no EUH phrases | ||||||||||||
| P: 273-280-301 + 310-501 | ||||||||||||
| MAK | Switzerland: 1 mg m −3 (measured as inhalable dust ) | Switzerland: 0.2 mg m −3 (measured as inhalable dust ) | ||||||||||
Web links
- CDC: HEXACHLOROCYCLOHEXANE (PDF; 623 kB)
Individual evidence
- ↑ State Institute for Environmental Protection Baden-Württemberg : Substance report hexachlorocyclohexane (HCH). 1st edition, 1993 ( pdf, 7.37 MB ).
- ^ Residues of pesticides in the soil (PDF; 978 kB).
- ↑ Bernd Pfaffenberger, Ingo Hardt, Heinrich Hühnerfuss, Wilfried A. König , Gerhard Rimkus, Alexandra Glausch, Volker Schurig and Jürgen Hahn: Enantioselective degradation of α-hexachlorcyclohexane and cyclodiene insecticides in roe-deer liver from different regions in Germany , Chemosphere 29 ( 1994) 1543-1554.
- ↑ a b Entry on alpha-1,2,3,4,5,6-hexachlorocyclohexane in the GESTIS substance database of the IFA , accessed on February 22, 2017(JavaScript required) .
- ↑ a b c Entry on beta-1,2,3,4,5,6-hexachlorocyclohexane in the GESTIS substance database of the IFA , accessed on February 22, 2017(JavaScript required) .
- ↑ a b c d Entry on lindane in the GESTIS substance database of the IFA , accessed on February 22, 2017(JavaScript required) .
- ↑ a b c Entry on delta-1,2,3,4,5,6-hexachlorocyclohexane in the GESTIS substance database of the IFA , accessed on February 22, 2017(JavaScript required) .
- ↑ CR Ganellin, David J. Triggle; Dictionary of pharmacological agents, ISBN 978-0412466304 .
- ↑ Data sheet BHC (mixture of hexachlorocyclohexanes) from Sigma-Aldrich , accessed on April 3, 2011 ( PDF ).
- ↑ Swiss Accident Insurance Fund (Suva): Limits - Current MAK and BAT values (search for 319-84-6 or α-hexachlorocyclohexane ), accessed on March 4, 2020.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 319-85-7 or β-hexachlorocyclohexane ), accessed on March 4, 2020.


