Brevetoxins
Brevetoxins (Ptychodiscus toxins, PbTx ) are neurotoxic algae poisons of the dinoflagellates of the species Karenia. The toxins are named after Karenia brevis (formerly Gymnodinium breve and Ptychodiscus brevis ). Brevetoxins are tasteless, odorless, acid and heat stable (up to 300 ° C).
Occurrence and meaning
Karenia brevis is a marine alga and part of the phytoplankton of tropical regions and is regularly observed in the Caribbean and especially in the Gulf of Mexico . Here it occurs as one of the causes of “ red tides ”, so-called red tides (red algae blooms ), which are formed by the mass reproduction of these algae. In the course of such “red tides”, there is repeated mass death of fish and animals of higher trophic levels (e.g. dolphins ) caused by toxic metabolic products of the algae, especially brevetoxins. In addition to the toxic effect, however, the reduction in the oxygen content, which is caused by the high density of phytoplankton on the water surface, is also involved in the mass extinction.
Brevetoxins can accumulate within the food chain. This leads to toxin-contaminated fish, mussels and crustaceans finding their way to humans as the end link of such food chains. In humans, the poisoning is known as Neurotoxic Shellfish Poisoning ( NSP ).
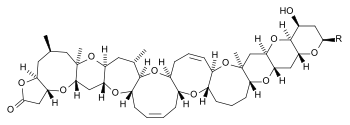
Structure and properties
Brevetoxins belong to the group of reduced non-aromatic polyketides . It is a polyether , the presence of tetrahydrofuran and / or tetrahydropyran rings is characteristic of these polyketides. They also have several ether bonds. Ten different brevetoxins are currently known, which can be divided into groups A and B. Brevetoxins A have ten five- to nine-membered rings, B eleven five- to eight-membered rings.
| Brevetoxin A | Brevetoxin B | |
|---|---|---|
| Structural formula | ||
| Subtypes |
|
|
Other brevetoxins:
- Brevetoxin-5 (PbTx-5): like PbTx-3, but the hydroxy group in position 38 is acetylated
- Brevetoxin-6 (PbTx-6): like PbTx-2, instead of the double bond 27-28 there is an epoxide
The structure elucidation as well as the total synthesis of these toxins was no easy undertaking. In 1981, the structure of brevetoxin B was the first of these toxins to be clarified; In 1995 Nicolaou et al. its total synthesis. The structure of brevetoxin A was clarified in 1986 and in 1998, again by Nicolaou et al. , completely synthesized for the first time.
toxicity
Brevetoxins are neurotoxins that cause irritation of the mucous membranes and the respiratory tract. The effects are similar to those of Ciguatoxin , but they are less toxic. It binds to voltage-dependent sodium channels and thus leads to a depolarization of nerve and muscle cells and ultimately to the transmission of stimuli and activation of these cells. In addition, they prolong the depolarization phase by inhibiting the inactivation of the sodium channel.
There is a risk of poisoning not only when bathing (especially when inhaling aerosols containing toxins ). In the restaurants, neurotoxic can with brevetoxins contaminated fish shellfish poisoning ( Neurotoxic Shellfish Poisoning , NSP) cause. In addition to gastrointestinal complaints, tingling of the lips and extremities, dizziness and coordination disorders occur. The heartbeat becomes slower ( bradycardia ). Hot-cold paresthesia are also possible. Due to the irritation of the respiratory tract and the toxicity to the body's own defenses ( immune system ), there is a risk of worsening symptoms , especially in people with respiratory diseases (e.g. asthma , bronchitis ).
Poisoning with brevetoxin is considered relatively harmless and does not cause permanent damage. In-vitro studies by John Ramsdell of the National Oceanic and Atmospheric Administration ( NOAA ) in Charleston / South Carolina showed that one of the toxins examined can very well cause permanent damage. It binds to DNA and forms DNA adducts that can cause mutations and errors in cell division. This is how they are classified as possibly carcinogenic . According to NOAA, however, it is too early to be able to say for sure, as there is a possibility that the body's own repair enzymes will correct the defects.
The substance Brevenal is an antagonist of brevetoxins. It binds to another point of the sodium channel and thereby displaces the brevetoxin. This inhibits the toxic effect of the toxin. Brevenal is also produced by Karenia brevis .
Brevetoxins are also a strong fish poison ( ichthyotoxin ).
Related links
The yessotoxins (YTX) are a group of lipophilic, sulfur-containing polyketides that are related to ciguatoxins and are similar to brevetoxins. They are produced by a wide variety of dinoflagellates, particularly Lingulodinium polyedrum and Gonyaulax spinifera . When environmental conditions encourage the growth of YTX-producing dinoflagellates, the toxins accumulate in the food chain. This leads to toxin-contaminated fish, mussels and crustaceans finding their way to humans as the end link of such food chains. In humans, the poisoning is known as Diarrhetic Shellfish Poison (DSP) . The IC 50 value of various YTX derivatives is approximately 0.5 nM.
See also
Individual evidence
- ↑ a b c d L. E. Fleming, B. Kirkpatrick, LC Backer, CJ Walsh, K. Kidneyberg, J. Clark: Review of Florida Red Tide and Human Health Effects . In: Harmful algae . 10, No. 2, 2011, pp. 224-233. doi : 10.1016 / j.hal.2010.08.006 .
- ^ A b c d Jean-Michel Kornprobst: Encyclopedia of Marine Natural Products . Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany 2014, ISBN 978-3-527-33585-5 , p. 246 , doi : 10.1002 / 9783527335855 ( wiley.com [accessed June 11, 2019]).
- ^ A b D. G. Baden: Brevetoxins: unique polyether dinoflagellate toxins . In: The FASEB Journal . 3, No. 7, 1989, pp. 1807-1817.
- ^ A b c d R. H. Pierce, MS Henry: Harmful algal toxins of the Florida red tide (Karenia brevis): natural chemical stressors in South Florida coastal ecosystems . In: Ecotoxicology (London, England) . 17, No. 7, 2011, pp. 623-631. doi : 10.1007 / s10646-008-0241-x .
- ↑ Yong-Yeng Lin, Martin Risk, Sammy M. Ray, Donna Van Engen, Jon Clardy, Jerzy Golik, John C. James, Koji Nakanishi: Isolation and Structure of Brevetoxin B from the “Red Tide” Dinoflagellate Ptychodiscus brevis (Gymnodinium breve ). In: Journal of the American Chemical Society. Vol. 103, No. 22, 1981, pp. 6773-6775, doi: 10.1021 / ja00412a053 .
- ^ KC Nicolaou , EA Theodorakis, FPJT Rutjes, J. Tiebes, M. Sato, E. Untersteller, X.-Y. Xiao: Total Synthesis of Brevetoxin B. 1. CDEFG Framework. In: Journal of the American Chemical Society. Vol. 117, No. 3, 1995, pp. 1171-1172, doi: 10.1021 / ja00108a051 .
- ↑ KC Nicolaou, FPJT Rutjes, EA Theodorakis, J. Tiebes, M. Sato, E. Untersteller: Total Synthesis of Brevetoxin B. 2. Completion. In: Journal of the American Chemical Society. Vol. 117, No. 3, 1995, pp. 1173-1174, doi: 10.1021 / ja00108a052 .
- ↑ KC Nicolaou, C.-K. Hwang, ME Duggan, DA Nugiel, Y. Abe, K. Bal Reddy, SA DeFrees, DR Reddy , RA Awartani, SR Conley, FPJT Rutjes, EA Theodorakis: Total Synthesis of Brevetoxin B. 1. First Generation Strategies and New Approaches to Oxepane Systems. In: Journal of the American Chemical Society. Vol. 117, No. 41, 1995, pp. 10227-10238, doi: 10.1021 / ja00146a008 .
- ↑ KC Nicolaou, EA Theodorakis, FPJT Rutjes, M. Sato, J. Tiebes, X.-Y. Xiao, C.-K. Hwang, ME Duggan, Z. Yang, EA Couladouros, F. Sato, J. Shin, H.-M. He, T. Bleckman: Total Synthesis of Brevetoxin B. 2. Second Generation Strategies and Construction of the Dioxepane Region [DEFG]. In: Journal of the American Chemical Society. Vol. 117, No. 41, 1995, pp. 10239-10251, doi: 10.1021 / ja00146a009 .
- ↑ KC Nicolaou, FPJT Rutjes, EA Theodorakis, J. Tiebes, M. Sato, E. Untersteller: Total Synthesis of Brevetoxin B. 3. Final Strategy and Completion. In: Journal of the American Chemical Society. Vol. 117, No. 41, 1995, pp. 10252-10263, doi: 10.1021 / ja00146a010 .
- ↑ Y. Shimizu, H.-N. Chou, H. Bando, G. Van Duyne, JC Clardy: . In: J. Am. Chem. Soc. 108: 514 (1986).
- ↑ KC Nicolaou, Zhen Yang, Guo-qiang Shi, Janet L. Gunzner, Konstantinos A. Agrios, Peter Gärtner: Total synthesis of brevetoxin A. In: Nature . Vol. 392, No. 6673, 1998, pp. 264-269, doi: 10.1038 / 32623 .
- ↑ Peter Nuhn , Ludger Wessjohann: Naturstoffchemie. Microbial, vegetable and animal natural substances. 4th, revised edition. Hirzel, Stuttgart 2006, ISBN 3-7776-1363-0 , p. 335.
- ↑ A. Tubaro, V. Dell'ovo, S. Sosa, C. Florio: Yessotoxins: A Toxicological Overview . In: Toxicon . 56, No. 2, 2010, pp. 163-172. doi : 10.1016 / j.toxicon.2009.07.038 . PMID 19660487 .
- ↑ MDA Howard, M. Silver, RM Kudela: Yessotoxin detected in mussel ( Mytilus californicus ) and phytoplankton samples from the US west coast . In: Harmful Algae . 7, No. 5, 2008, pp. 646-652. doi : 10.1016 / j.hal.2008.01.003 .