Levosalbutamol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
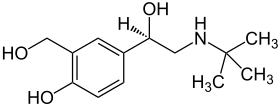
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Levosalbutamol | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | ||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Levosalbutamol is a drug from the group of β 2 -sympathomimetics , which is used as a bronchospasmolytic in bronchial asthma and chronic obstructive bronchitis . When administered by inhalation, levosalbutamol causes a rapid onset and long-lasting relaxation of the smooth muscles in the bronchi . The effect is based on the stimulation of β 2 -adrenoceptors .
Stereoisomerism
Levosalbutamol is the international non-proprietary name (INN) of the enantiomerically pure ( R ) - isomer of salbutamol , which is chiral , i.e. contains a stereocenter. Levosalbutamol is the effective enantiomer (= eutomer ) of salbutamol.
The racemate salbutamol, on the other hand, consists of the two enantiomers , ( R ) -2- ( tert -butylamino) -1- (4-hydroxy-3-hydroxymethylphenyl) ethanol and ( S ) -2- ( tert -butylamino) -1- ( 4-hydroxy-3-hydroxymethylphenyl) ethanol, in a ratio of 1: 1.
Manufacturing
Several multi-step syntheses for levosalbutamol are described in the literature.
Finished medicinal products
Ventoplus ( AR ), Xopenex ( USA )
The inhalation solutions contain levosalbutamol hydrochloride or levosalbutamol hemitartrate, which are readily soluble in water.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Axel Kleemann , Jürgen Engel, Bernd Kutscher and Dieter Reichert: Pharmaceutical Substances , Thieme-Verlag Stuttgart, 5th edition (2009), pp. 800–802 ISBN 978-3-13-558405-8 ; also online with biannual additions and updates.