Calcium hydride
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
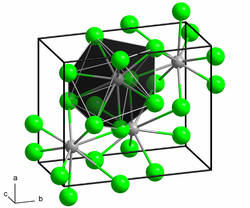
|
||||||||||||||||
| __ Ca 2+ __ H - | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Calcium hydride | |||||||||||||||
| other names |
|
|||||||||||||||
| Ratio formula | CaH 2 | |||||||||||||||
| Brief description |
colorless hexagonal prisms |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 42.1 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.9 g cm −3 |
|||||||||||||||
| Melting point |
1000 ° C |
|||||||||||||||
| boiling point |
Decomposition> 600 ° C |
|||||||||||||||
| solubility |
decomposes in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Calcium hydride is the metal hydride of calcium .
presentation
Calcium hydride is produced by passing hydrogen over calcium at 400 ° C:
properties
Calcium hydride occurs in two polymorphic crystal forms. The low-temperature form A with an orthorhombic crystal structure changes into the high-temperature form B at 780 ° C. This then melts at 1000 ° C.
use
Since it decomposes when it comes into contact with water, generating a lot of hydrogen, calcium hydride serves as a storage substance in order to be able to produce hydrogen regardless of location:
Around 1 m 3 of hydrogen is produced per kilogram of calcium hydride .
In addition, calcium is the so-called Hydrimet method used metal oxides (e.g., vanadium pentoxide , zirconium dioxide , titanium dioxide or sodium peroxide ) to elemental to metal reduce . With processing temperatures between 600 and 1,000 ° C, this is done under relatively gentle conditions. The hydrogen atmosphere that forms during the reaction also protects the metal that is formed:
Because of its water-drawing properties, calcium hydride is also used as a drying agent .
It is also used to measure the residual moisture in plastics.
Individual evidence
- ↑ a b c Entry on calcium hydride. In: Römpp Online . Georg Thieme Verlag, accessed on September 23, 2014.
- ↑ a b c Calcium hydride data sheet (PDF) from Merck , accessed on January 19, 2011.
- ^ A b I. Barin: Thermochemical Data of Pure Substances , Wiley-VCH, 3rd edition 1997, ISBN 978-3527287451 , p. 296.
- ↑ a b Entry on calcium hydride in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Entry on Calcium hydride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .



