Tetraethyl lead
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Tetraethyl lead | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 20 Pb | ||||||||||||||||||
| Brief description |
colorless, easily mobile, sweet-smelling, poisonous liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 323.45 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.65 g cm −3 |
||||||||||||||||||
| Melting point |
−136 ° C |
||||||||||||||||||
| boiling point |
200 ° C (decomposition) |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.519 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Authorization procedure under REACH |
of particular concern : toxic for reproduction ( CMR ) |
||||||||||||||||||
| MAK |
0.05 mg m −3 (calculated as lead) |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
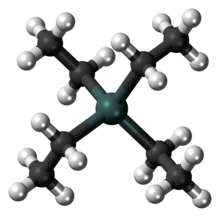
Tetraethyl lead ( TEL , of English t etra e thyl l ead ) is a lead-organic compound with the molecular formula C 8 H 20 Pb ( structural formula (C 2 H 5 ) 4 Pb), in which four ethyl tetrahedrally bonded to a central lead atom. It is a colorless, viscous liquid with a pleasant, sweet odor that is stable in air and water.
The German chemist Carl Löwig first reported on the production of tetraethyl lead in 1853. It gained technical and commercial importance from the 1920s, when Thomas Midgley discovered its effectiveness as an anti-knock agent for petrol in the General Motors research laboratory . The Standard Oil of New Jersey , which had the patent on the production, and General Motors, which owned the patent application, then founded in 1924 the Ethyl Corporation that manufactured and marketed tetraethyl lead.
Tetraethyl lead acts as an anti- knock agent , as it intercepts the hydroperoxyl radicals that arise when hydrocarbons are burned in the engine, thereby inhibiting a branched chain reaction and thus preventing knocking . Due to its good price-effectiveness ratio, it has been used worldwide as an anti-knock agent. Its secondary function was to limit the wear and tear on valves and valve seats in engines. The use of tetraethyl lead in gasoline has emitted millions of tons of lead in the United States alone between its introduction and its ban.
Tetraethyl lead is absorbed into the body by inhalation , through absorption through the skin or by ingestion . It is lipophilic , toxic and even small amounts of it can lead to severe lead poisoning . The ultimately toxic molecule is the triethyl lead ion, which damages the central nervous system and can trigger toxic psychosis or paralysis . The lead oxide and halide particles produced when tetraethyl lead is burned in the engine are emitted with the exhaust gas and absorbed with the air we breathe. They also damage the nervous system , especially that of children.
After poisoning and deaths initially occurred in the production of tetraethyl lead, the Surgeon General of the United States briefly banned its production and use in 1925. From the 1970s onwards, the United States restricted the use of tetraethyl lead under the Clean Air Act . In addition to protecting health, this should prevent the poisoning of three-way catalytic converters . In the Federal Republic of Germany , the lead content in gasoline was restricted to 0.15 grams per liter in 1976, and in 1988 the legislature banned normal leaded gasoline in Germany. The ban on leaded premium gasoline followed in 1996. The European Union and Switzerland banned leaded gasoline on January 1, 2000.
history
Tetraethyl lead is one of the organometallic alkyl compounds whose history began in 1760. In that year Louis Claude Cadet de Gassicourt synthesized the first compound of its kind, the cacodyl . Robert Wilhelm Bunsen and his students Carl Kolbe and Edward Frankland systematically investigated the cacodyl from 1836. Frankland deepened his research in this direction and in 1849 synthesized further organometallic alkyl compounds, diethyl zinc and dimethyl zinc .
Laboratory syntheses
Inspired by Frankland's studies, other chemists devoted themselves to this research area. For example, Carl Löwig , who succeeded Bunsen at the Chair of Chemistry at the University of Breslau in 1853 , reported in the same year on the preparation of the first organometallic acrylic lead compound. For their representation he used a lead - sodium alloy, which he converted with iodoethane . Extraction with diethyl ether gave it a colorless liquid which dissolved in diethyl ether and ethyl acetate , but not in water. Löwig could not present tetraethyl lead as a pure compound, since it decomposed during distillation.
Finally, George Bowdler Buckton , who at the time was assistant to August Wilhelm von Hofmann at the Royal College of Chemistry in London, reported on the first presentation of pure tetraethyl lead . To this end, Buckton converted diethyl zinc with an excess of lead (II) chloride . By distilling the reaction product in a slight vacuum, he succeeded in preparing pure tetraethyl lead. The reactions of tetraethyl lead with hydrogen chloride to form triethyl lead chloride , with sulfuric acid to form hexaethyldible disulfate ([(C 2 H 5 ) 3 Pb] 2 SO 4 ) and with potassium hydroxide to form hexaethyldible oxide ([(C 2 H 5 ) 3 Pb] 2 O) he also examined. The steam distillation of tetraethyl lead, which was later common in the industrial sector , was introduced by Frankland in 1879, who was also the first to propose the correct structural formula for tetraethyl lead.
Discovery of anti-knock behavior
In the chemistry laboratories of European universities, chemists synthesized many other organometallic alkyl compounds in the late 19th and early 20th centuries, and tetraethyl lead was just one of many. It was not foreseeable that this connection would become of great technical importance for solving the knocking problem from the 1920s . The phenomenon of knocking and its damaging effect on the engine was already described by Nicolaus Otto in 1862 in his comments on the work on his first four-stroke engine , a four-cylinder test machine:
"It ran in 1862 and was totally ruined in the same year by the violent bumps that occurred in it."
This knocking limited the increase in the efficiency of the gasoline engine, especially in the upper load range. A knock-resistant fuel allows for a higher compression ratio , which leads to higher fuel efficiency and better performance .
Charles Kettering , the inventor of the electric starter and founder of the Dayton Engineering Laboratories Company ( Delco ), was concerned with improving the thermal efficiency of internal combustion engines from around 1910 . He sensed that the solution to the knocking problem was of crucial importance and in 1916 commissioned his colleague Thomas Midgley to investigate it. Midgley then tested the anti-knock properties of iodine , aniline and thousands of other organic and inorganic substances . Although some of the tested fabrics showed useful anti-knock properties, they were ruled out because of other undesirable properties such as high cost or their tendency to corrode . The organometallic alkyl compounds diethyl selenide ((C 2 H 5 ) 2 Se) and diethyl telluride ((C 2 H 5 ) 2 Te) showed good anti-knock properties, but the latter in particular gave the exhaust gas a “satanic garlic odor”, according to Midgley.
General Motors (GM) took over Kettering's company in 1919 and turned it into the automotive company's research department, which Kettering led as Vice President . At that time, chemist Robert E. Wilson , Professor of Applied Chemistry at the Massachusetts Institute of Technology , was also working on research projects for GM. Based on the good anti-knock properties of the alkyl metal compounds, he directed Midgley's interest to a systematic investigation of this product group. In December 1921, tests were finally carried out with tetraethyl lead, which showed good anti-knock properties at a concentration of 0.025%. Tetraethyl lead was found to be 118 times more effective than aniline and about 1200 times more effective than ethanol, and was far superior to all other products tested.
Based on the work of Löwig, the first technical synthesis of tetraethyl lead on the basis of iodoethane took place, which however quickly turned out to be too expensive. DuPont then developed a process based on bromoethane , which, however, also turned out to be too expensive. In the meantime, GM is negotiating with Standard Oil of New Jersey to use tetraethyl lead in their products. The conditions offered by GM prompted Standard Oil to develop its own process based on inexpensive chloroethane. Standard Oil awarded the research work to Charles August Kraus , who developed the industrial process with his colleague Conrad Callis and patented it in 1923.
Because tetraethyl lead was much cheaper to manufacture by this process than by the GM and DuPont processes, Standard Oil Company of New Jersey, which held the manufacturing patent, and General Motors, which held the application patent, founded Ethyl Gasoline Corporation on August 18, 1924. In this company, the shareholders bundled the activities for the production, use and marketing of tetraethyl lead. The Ethyl Corporation hired DuPont to manufacture it because neither Standard Oil nor GM had any experience in the field.
During test drives and on engine test stands , it was found that tetraethyl lead could not be used without problems. The burned tetraethyl lead left a layer of lead (II) oxide in the combustion chamber and on the spark plugs . Thomas Alwin Boyd , a Midgley employee, discovered that the addition of 1,2-dibromoethane prevented these deposits. This additive formed the more volatile lead (II) bromide , which was emitted with the exhaust gas.
Since bromine was scarce and expensive, GM's research department worked with Dow Chemical to develop a process for extracting bromine from seawater. For this purpose, chlorine was fed into the seawater, which oxidized the bromides present there and thus released bromine. Plants in Kure Beach , North Carolina , and Freeport , Texas , supplied the bromine required for the bromination of ethene . Curiously, this process also goes back to Carl Löwig, who in 1825 obtained bromine by introducing chlorine into Bad Kreuznach brine water .

On February 2, 1923, the Refiners Oil Company sold the first liters of tetraethyl lead to a motorist in Dayton, Ohio . Using a so-called "ethylizer", a hand pump, the tetraethyl lead was pumped into the gasoline. By August 1923, around 30 Ethylizers were in use, the number rose to over 17,000 by October 1924. The top three places in the Indianapolis 500 auto race in 1923 went to racing cars that had used tetraethyl lead-infused gasoline, a result that was highlighted in the press. About 18 months later, leaded gasoline sales were already 380 million liters per year.
First ban on tetraethyl lead
Workers involved in the manufacture of tetraethyl lead soon began to behave strangely. The workers would have hallucinations and snap at imaginary butterflies that were supposed to be flying around them or perching on their bodies. Tetraethyl lead was then nicknamed "The loony gas" (German: "The crazy gasoline"), the production building was nicknamed "House of Butterflies" (German: "House of Butterflies"). In the autumn of 1924 the condition of the workers deteriorated rapidly, 32 were hospitalized and 5 of them died.
The Office of Chief Medical Examiner of the City of New York (OCME) under Charles Norris led investigations into the deaths. Alexander O. Gettler , an American biochemist and pioneer of forensic toxicology , found high levels of lead in the bodies of the dead, the cause of death ultimately being lead poisoning . This led to a temporary ban on tetraethyl lead as a gasoline additive in New York City , New Jersey and Philadelphia, among others . Several European states joined the ban in 1925. Switzerland supplemented the food ordinance and banned the use of selenium and tellurium compounds in addition to lead.
At a 1925 Surgeon General hearing on the dangers of tetraethyl lead, Yandell Henderson , an American physiologist from Yale University, and David Lynn Edsall of Harvard Medical School spoke out against the use of tetraethyl lead. They predicted creeping poisoning from the lead emitted.
"Conditions would grow worse so gradually and development of lead poisoning will come on ... insidiously ... before the public and the government awaken to the situation."
"Conditions would gradually worsen, and lead poisoning would develop ... insidiously ... before the public and government became aware of the situation."
The supporters, on the other hand, called tetraethyl lead a “gift from God”, which significantly extends the scarce oil reserves. A ban would only be justified if there was evidence of a threat to public health. The US federal government finally lifted the ban on tetraethyl lead in 1926.
Triumphant advance of tetraethyl lead
The development of the modern and powerful engine was largely dependent on the availability of high quality gasoline. The addition of tetraethyl lead appeared to be the most economical way to make these high quality gasolines and was considered one of the most important advances in the fuel and automotive industries . Alfred P. Sloan , who succeeded Pierre Samuel du Pont as President of GM in 1923 , took advantage of the availability of high-octane fuels to build automobiles with higher speed and power. This allowed GM a stronger differentiation between the group brands, which allowed the customer to climb a "ladder of success". The customer drove Chevrolet as an entry-level brand, and then moved up to Cadillac via Pontiac , Oldsmobile and Buick . With this strategy, GM succeeded in ousting Ford out of its dominance of the market and in becoming the largest American automobile company.
The American public enthusiastically celebrated Midgley's discovery, and the academic world honored him with the William H. Nichols Medal , the Priestley Medal , the Willard Gibbs Medal , the Perkin Medal and two honorary doctorates . The American Chemical Society elected the mechanical engineer Midgley, who had also developed the chlorofluorocarbons , as its president in 1944. On the occasion of the presentation of the Perkin Medal to Midgley, the New York Times wrote :
“Midgley's work resulted in the creation of the entire ethyl gasoline industry with all that it implies - use of higher compression engines, greater flexibility of automobile operation and other advances. Tetraethyllead in motor fuels adds fifty times as much horsepower annually to American civilization as that which will be supplied by Boulder Dam ”
“Midgley's work led to the creation of the entire ethyl gasoline industry and all that goes with it - the use of higher compression engines, greater flexibility in automotive operations, and other advances. Tetraethyl lead in motor fuels provides American civilization with 50 times the power that Boulder Dam will provide annually. "
License to IG Farben
In the run-up to the Second World War , tetraethyl lead became important in the war economy. By adding tetraethyl lead, aviation gasoline with an octane rating of 100 and higher could be produced. While the Luftwaffe of the Wehrmacht used fuels with an octane rating of 87 to 90, the use of high-octane gasoline led to a significant improvement in most of the aircraft's combat-related performance factors. These included increased engine power when climbing , lower fuel consumption and increased top speed.
The IG Farben acquired in 1935 a license for the production of tetraethyl lead to be able to produce so knock firmer aviation fuel. The United States Department of War examined the matter and did not object to the joint venture.
After the establishment of Ethyl GmbH by IG Farben, Standard Oil of New Jersey and GM, two tetraethyl lead plants were built and an aviation fuel contract was signed with the Nazi government on June 10, 1936. One of these tetraethyl lead plants with an annual production of 1200 tons was built in Gapel in 1936 , the other was opened in Frose in 1938/39 and had an annual output of 3600 tons.
Mass motorization after the Second World War
From the 1950s, with the economic miracle and the onset of mass motorization, the demand for high-octane gasoline and thus for tetraethyl lead rose sharply in Germany and Central and Western Europe , although Switzerland did not lift a ban on its use in motor gasoline until 1947. In the United States, the number of automobiles per 1,000 population increased from 222 to 545 between 1945 and 1970, and to 711 in 1980, and the demand for tetraethyl lead increased accordingly. In 1983, Germany consumed around 4,600 tons of lead in the form of tetraethyl lead.
The Esso Tiger and the “Put the tiger in the tank!” Slogan, which was invented in 1959, promoted Esso petrol mixed with tetraethyl lead in the 1960s and 1970s. The successful campaign increased the company's sales significantly, which is why Time Magazine declared 1964 the year of the tiger.
At the same time, the environmental pollution from automobile traffic and the associated emissions of carbon monoxide , hydrocarbons , nitrogen oxides and sulfur dioxide increased . The smog caused by road traffic in large US cities in the 1960s and 1970s such as New York City and Los Angeles heightened public awareness of the environment. The thick smog in Los Angeles even sparked rumors of a Japanese gas attack. President Lyndon B. Johnson and members of Congress began drafting federal laws to regulate air pollution in the United States. A significant reduction in emissions should be achieved through the use of three-way catalytic converters. To do this, it was necessary to do without tetraethyl lead across the board.
The Muskie hearings
The Senator from Maine , Edmund Muskie , initiated in 1966 a committee for hearings on the Clean Air Act , a US statutory provision to air pollution . Muskie focused the hearings on the health effects of lead emissions from the burning of leaded gasoline. At the hearing, then Surgeon General of the United States , William Stewart, the operational director of the United States Public Health Service , expressed concern about the health effects of lead exposure on children and pregnant women. Studies have shown that lead exposure has been linked to the incidence of intellectual disability in children. The focus of the hearing was the scientific debate between Robert A. Kehoe and Clair Cameron Patterson .
Robert A. Kehoe was the chief medical officer of Ethyl Corporation and director of the UC Kettering Laboratory of Applied Physiology, funded by GM, DuPont, and the Ethyl Corporation. He was considered the leading expert on the toxicological effects of lead. Kehoe had examined the blood lead content of Mexican farmers and found values that were comparable to a US American, inner-city group, although the farmers were hardly exposed to the influence of lead emissions from car exhaust. From this, Kehoe concluded that lead is a natural part of body chemistry. The correct explanation for the elevated blood lead levels, however, was that these farmers consumed food that was contaminated by lead-containing clay dishes. Kehoe was also of the opinion that humans had achieved a biological adaptation to lead in the course of evolution. His work and activity led to the acceptance of a system of voluntary self-regulation by the lead industry as a model for assessing environmental impact .
Clair Patterson was an American geochemist who developed uranium-lead dating to calculate the age of the earth and was awarded the VM Goldschmidt Award of the Geochemical Society for this. During his investigations, he found that lead from the environment falsified his measurements and that complex methods to control sample contamination were required in order to achieve reliable results when determining the ratio of lead isotopes . His work eventually showed that there has been a sharp increase in lead pollution since the introduction of tetraethyl lead and that the natural value was much lower. An evolutionary adaptation to lead by humans was thus excluded. Patterson revealed at the hearing not only that Kehoe's data were incorrect and that a large number of people were sick due to the lead exposure, but he also attacked the way the Public Health Service worked.
“It is not just a mistake for public health agencies to cooperate and collaborate with industries in investigating and deciding whether public health is endangered; it is a direct abrogation and violation of the duties and responsibilities of those public health organizations. "
“It is not just a mistake for public health authorities to partner and collaborate with industries to investigate and determine whether public health is at risk; it is a direct override and a violation of the duties and responsibilities of these public health organizations. "
Patterson's appearance and support from Senator Muskie changed public perceptions of the dangers of tetraethyl lead. As a result, awareness of the risks to public health increased significantly. Muskie also supported the concept of the dose-response curve introduced by Patterson , in which acute lead poisoning is only one point in a spectrum of reactions of the human organism to the ingestion of lead. This concept henceforth played an essential role in regulating tetraethyl lead in gasoline. Patterson, who emerged victorious from the scientific confrontation with Kehoe, subsequently lost his contract with the Public Health Service as well as his contract with the American Petroleum Institute . Several members of the California Institute of Technology Board of Trustees asked his manager to be dismissed.
Exit
The three-way catalytic converters introduced in the 1970s changed the demands of both automobile and gasoline production. They reduced the emissions of hydrocarbons , carbon monoxide and nitrogen oxides in car exhaust, but required gasoline without the addition of tetraethyl lead. Deposits of lead oxides on the surface of the catalyst reduced the efficiency of the catalysts by deactivating the catalytically active noble metals . The introduction of catalytic converters and the development of unleaded petrol led to the introduction of hardened valve seats by most automobile manufacturers.
Based on the scientific evidence of the hazards posed by tetraethyl lead to both health and the use of three-way catalysts, the U.S. Environmental Protection Agency issued regulations to reduce lead levels in 1973 and enacted them in 1976. The European and Japanese environmental protection agencies followed the US model. From the mid-1980s, more and more petrol stations in Germany offered unleaded petrol , initially parallel to conventional leaded fuels. From June 1984, unleaded petrol was available in Austria . With the increasing spread of catalytic converters that rely on “unleaded” gasoline, it was available practically everywhere after a few years. Regular leaded gasoline was banned in Germany in 1988; the ban on leaded premium gasoline followed in 1996. AK Chemie in Biebesheim am Rhein (a subsidiary of Octel Germany, which in turn was owned by BP , Caltex , Mobil Oil and Shell ), was the only company in Germany to produce tetraethyl lead; the company stopped production in the early 1990s.
The European Union and Switzerland banned leaded petrol on January 1, 2000. On December 19, 2012, the EU classified tetraethyl lead as a substance of very high concern . It is still classified as a bioaccumulating substance and a substance with problematic environmental properties.
China banned leaded gasoline around 2001. In other countries and regions the ban on tetraethyl lead came later, in 2002, 82 countries still used tetraethyl lead as a gasoline additive. Algeria is the last country to use tetraethyl lead for petrol. Innospec , formerly known as Octel Corporation, is the last manufacturer in the world to produce tetraethyl lead and continues to export it from the UK to Algeria.
The US NASCAR racing series used leaded gasoline with 110 octane until 2008 with a special permit. For small aircraft, leaded gasoline is still offered and used under the name AvGas . According to the US Environmental Protection Agency (EPA) , AvGas is the largest source of lead emissions. Highly leaded AvGas variants are no longer available, but AvGas 100LL with an octane number of 100 and a lead content of 0.56 grams of lead per liter is sold nationwide as an aviation fuel for the operation of aircraft with reciprocating engines . In Switzerland and Austria, AvGas 100 LL is also the standard quality for aviation fuel.
Manufacturing
The excellent effectiveness of tetraethyl lead as an anti-knock agent and its use as a gasoline additive from 1923 onwards led to an enormous number of studies on its synthetic methods and industrial production. The industrial production was mainly based on a variant of the synthesis route already used by Löwig using a lead-sodium alloy and an electrochemical process.
Industrial manufacture
The industrial production of tetraethyl lead takes place through a solid-liquid reaction of a sodium - lead alloy with chloroethane at a temperature of 50 to 75 ° C, also known as the Kraus-Callis process after Charles August Kraus and Conrad C. Callis . The lead is melted with 10% sodium under a protective gas . The alloy is then mechanically crushed to a grain size of 5 to 6 millimeters and then reacted with chloroethane in an autoclave at around 50 to 75 ° C. To avoid an increase in pressure in the autoclave, the chloroethane used must be free of impurities such as vinyl chloride . Aluminum chloride acts as a catalyst. In a batch with 1350 kilograms of the lead-sodium alloy and 590 kilograms of chloroethane, around 400 kilograms of tetraethyl lead are formed. The reaction time for such a batch size is about 8 hours.
Tetraethyl lead is then distilled off by means of steam distillation and then dried. The resulting metallic lead is converted back into a lead-sodium alloy with sodium. The yield is about 88% based on sodium. Typical by-products are hydrocarbons such as n-butane , which are formed by sodium-induced coupling reactions .
In the patent literature, among other things, ethyl acetate, water and various oxygen-containing hydrocarbons are specified as promoters . Since the reaction may include a reduction stage , these substances could serve as hydrogen suppliers.
About 60% of US production of metallic sodium and 85% of chloroethane were used in the synthesis of tetraethyl lead by this process. This process accounted for 65% of chloroethane production in Europe. Along with the ban on leaded gasoline, the demand for metallic sodium and chloroethane decreased significantly.
The Nalco process also produced tetraethyl lead on an industrial scale. The electrolysis was a solution of a Ethylmagnesiumgrignardreagenz and an ethyl halide at a lead anode and a magnesium - cathode . The alkyl radicals formed during the anode reaction react with the electrode material to form tetraethyl lead. The overall response is:
Nalco started production in 1964 and had a market share of 11.8% in the 1970s. The rest of the market was shared by Ethyl Corporation (33.5%), DuPont (38.4%) and PPG Industries (16.2%).
Other ways of representation
Tetraethyl lead is usually prepared by oxidative addition to metallic lead or to lead (II) compounds with subsequent disproportionation . The use of lead (IV) compounds for synthesis is also possible.
The reaction of lead (II) chloride with an ethyl Grignard compound leads to the unstable intermediate product diethyl lead:
This reacts by disproportionation to tetraethyl lead and elemental lead:
Hexaethyldiblei is obtained as a by-product .
Tetraethyl lead can be prepared by reacting lead (IV) chloride with Grignard compounds or with triethylaluminum . Because of the unstable nature of lead (IV) chloride, this is not a common practice.
Tetraethyl lead is also formed by the reduction of bromoethane on lead cathodes in propylene carbonate solutions with tetraalkylammonium salts as supporting electrolytes.
Tetraethyl lead can also be produced by the reaction of diethyl zinc with lead (II) chloride.
Tetraethyl lead can be obtained by salt metathesis of triethyl lead chloride by exchanging the chloride and ethyl ligands.
Tetraethyl lead can also be produced by hydroplumbing , for example by reacting triethyl plumban with ethene . Triethylplumban is an unstable compound that can be obtained by reacting triethyl lead chloride with sodium borohydride .
Tetraethyl lead can also be prepared by reacting lead (II) chloride with ethyl lithium to give triethyl lead lithium and then reacting with chloroethane.
properties
Physical Properties
Tetraethyl lead is a colorless, oily, volatile liquid with a density of 1.653 g cm³ at 20 ° C. The melting point is −136 ° C and the boiling point is 200 ° C (with decomposition), the flash point is approx. 80 ° C. The molar mass is 323.45 g mol −1 .
Observations of the melting point of tetraethyl lead indicated that the compound can crystallize in at least six different forms, with their melting points in a range of a few degrees. It is believed that the unusual polymorphism of tetraethyl lead is due to the size of the central atom, which allows the ethyl groups to have a form of rotational isomerism.
Molecular Properties
The carbon-lead bond is a purely covalent σ bond . In organic compounds, lead is sp 3 hybridized , the ethyl ligands are arranged tetrahedral on the central atom. The length of the Pb – C bond in tetraethyl lead is 229 picometers (pm), the dissociation energy is 226 kilojoules per mol (kJ / mol).
Chemical properties
Tetraethyl lead is soluble in many organic solvents, but hardly soluble in dilute acids or alkalis. The solubility in water is 0.29 mg / l at 25 ° C. Since the four ethyl groups are arranged tetrahedrally around the lead atom, the positive (Pb) and the added negative centers of charge (C 2 H 5 ) coincide. Since the molecule is also uncharged, it is non-polar and therefore lipophilic . This results in its good solubility in non-polar and very poor solubility in polar solvents, for example water.
While many purely inorganic lead salts are in oxidation level II, in the chemistry of alkyl lead compounds the oxidation level IV predominates. Alkyl lead compounds in oxidation level II disproportionate easily into metallic lead and lead (IV) alkyls.
Tetraethyl lead burns with an orange flame that is bright green around the edges. It reacts violently when iodine and bromine are added . The chemistry is dominated by the weak Pb-C bond. Free ethyl radicals could be detected by pyrolysis of tetraethyl lead at low pressures in a stream of inert gas in a glass tube. For this purpose, the ethyl radicals were converted into tetraethyl lead using a lead mirror that had previously been deposited on the cold part of a glass tube. This conversion is considered to be the first evidence of the existence of simple aliphatic free radicals.
Treatment of the tetraethyl lead with silver nitrate in ethyl acetate solution followed by the addition of aqueous potassium hydroxide leads to the formation of bis-triethyl lead dioxide ([(C 2 H 5 ) 3 Pb] 2 O). In air, this reacts with the absorption of carbon dioxide to form bis-triethyl lead carbonate. The reaction of tetraethyl lead with hydrochloric acid or aqueous potassium bromide solution leads to the formation of the triethyl lead halides (C 2 H 5 ) 3 PbCl or (C 2 H 5 ) 3 PbBr as crystalline solids. Tetraethyl lead reacts with dry sulfur dioxide in inert solvents with the insertion of SO 2 into the Pb-C bond to form organic lead sulfinates .
Tetraethylbleibildet forms octakis (3,4-dimethylphenylthio) naphthalene inclusion complexes . The crystal structure analysis of the tetraethyl lead adduct shows that the adduct crystallizes in the cubic space group Pn 3 , the ethyl groups of the tetraethyl lead being highly disordered.
use
In the 1950s a solution called "Ethyfluid" was on the market. This consisted of tetraethyl lead (54.6%), 1,2-dibromoethane (36.4%), a blue anthraquinone dye (0.01%) and “halo wax oil”, 1-chloronaphthalene , 9%, which was used as a lubricant for the piston rings was intended. The 1,2-dibromoethane was partly replaced by 1,2-dichloroethane. The production volume of tetraethyl lead was already 30,000 tons in 1937, with which about 64 million liters of gasoline were lead, with an octane number increase of 5 points. The associated savings amounted to approximately 2.8 million liters of gasoline. The later developed "TEL Motor 33 Mix" contained about 57.5% tetraethyl lead, 17.6% dichloroethane, 16.7% dibromoethane, 7.0% (methylcyclopentadienyl) manganese tricarbonyl, 1.2% dye and lecithin or 4-tert- Butylphenol as an antioxidant .
| year | United States | Western world (excluding USA) |
|---|---|---|
| 1965 | 225,000 | 35,000 |
| 1966 | 247,000 | 36,000 |
| 1967 | 247,000 | 46,000 |
| 1968 | 262,000 | 48,000 |
| 1969 | 271,000 | 44,000 |
| 1970 | 279,000 | 47,000 |
| 1971 | 264,000 | 113,000 |
| 1974 | 250,000 | 125,000 |
| 1975 | 175,000 | 126,000 |
Anti-knock agent for petrol

clock 1: Sampling; Cycle 2: compression; Igniting and burning the mixture at top dead center; Measure 3: work; Measure 4: Eject
The efficiency of a gasoline engine increases with increasing compression ratio. However, the compression cannot be increased arbitrarily by the occurrence of the engine knock. Knocking occurs during combustion, at the end of the compression cycle and at the beginning of the work cycle after ignition by the ignition spark ; the ignition causes a flame front to spread in the combustion chamber, which normally ignites the entire mixture in a controlled manner. When knocking, the so-called end zones of the mixture are exposed to so much heat and pressure by the flame front that they ignite by themselves before they are reached by the flame front. The resulting, almost isochoric combustion of the residual gas leads to steep pressure gradients which spread as pressure waves in the combustion chamber and lead to a noise known as knocking or ringing. The pressure waves that occur during knocking can lead to material damage; The strong thermal loads can melt the metal of the cylinder and piston in places.
When tetraethyl lead is burned, lead oxide particles are formed, which offer a finely distributed heterogeneous surface. Hydroperoxide radicals that are adsorbed on this surface can no longer take part in radical chain reactions. The resulting reduced reaction speed is sufficient to suppress the spontaneous ignition of the previously unburned portion of the mixture and to eliminate knocking.
From the 1920s to the early 1960s, tetraethyl lead alone was used as an octane booster for petrol. Once in the 1960s, the technical problems in the manufacture of tetramethyl lead were overcome these organolead compound was also used in a mixture with tetraethyl lead, typically in the ratio 1: 1. Alternatively, by catalytic ligand substitution of the alkyl groups between tetraethyl and tetramethyl lead mixed methyl-ethyl -Lead compounds are synthesized. These were sold under the name “Lead Mix 75”, a product of ligand substitution of 75 mol% tetramethyl lead and 25 mol% tetraethyl lead.
- with n = 1 to 3
The boiling points of the tetraalkyl lead compounds are between 110 ° C for tetramethyl lead and 200 ° C for tetraethyl lead. This made it possible to specifically improve the knock resistance of certain boiling fractions. Gasoline contains almost 200 different aromatic and aliphatic hydrocarbons of different structure and molecular mass , from volatile butane to aromatics with twelve carbon atoms per molecule. The volatility of the various components influences the suitability of a fuel and must be adjusted according to the season. The proportions of the different boiling fractions can be determined by means of boiling analysis . The part that evaporates below 70 ° C easily forms a flammable mixture with air and promotes cold start behavior . On the other hand, too high a proportion of low boilers could lead to vapor bubbles in the fuel system in summer. Due to their boiling temperature, which is adjusted to the boiling fractions, the mixed tetraalkyl lead compounds often give better knock resistance than mixtures of tetramethyl and tetraethyl lead.
| Anti-knock agents | Boiling point in [° C] |
|---|---|
| Tetraethyl lead | 200 |
| Triethylmethyl lead | 179 |
| Diethyl dimethyl lead | 159 |
| Ethyl trimethyl lead | 137 |
| Tetramethyl lead | 110 |
It quickly became apparent that the use of tetraethyl lead led to the deposition of lead oxides on engine valves and spark plugs. The search for additives that could remove the lead oxides from the engine then began. Thomas Alwin Boyd found that 1,2-dibromoethane, when added to the tetraethyl lead containing fuel, prevented the formation of lead containing deposits. The 1,2-dibromoethane reacted with the poorly volatile lead oxide components to form low-melting lead halides such as lead (II) bromide , which has a melting point of 373 ° C. This is emitted as part of the exhaust gas.
The very first leaded gasoline sold contained brominated organic compounds known as “scavengers”. These were initially added to gasoline together with chlorinated organic compounds such as carbon tetrachloride . 1-chloronaphthalene was used intermittently. 1,2-dichloroethane has been used as a scavenger since the 1940s . A molar ratio of Pb: Cl: Br of 1: 2: 1 is aimed for for motor gasoline.
In 1957, Ethyl Corporation developed (methylcyclopentadienyl) manganese tricarbonyl (MMT), another metal-based anti-knock agent. From the 1970s, especially in the United States and Canada , this was added to gasoline as an amplifier for tetraethyl lead. The amounts added were in the range of 8.3 milligrams of manganese per liter for motor gasoline in the USA. In Canada the gasoline had an average content of 12 milligrams of manganese per liter.
After the ban on tetraethyl lead in Canada, it was completely replaced by MMT as an anti-knock agent between 1990 and 2003. In Canada, refineries voluntarily stopped using MMT in 2003. California banned manganese additives in gasoline in 1976, New Zealand in 2002, in Japan it was not used. In Germany, the use of other metal compounds, including (methylcyclopentadienyl) manganese tricarbonyl, was forbidden by the gasoline lead law.
Protection of the valve seats
The lead oxides produced by the combustion of tetraethyl lead are deposited between the valve and the valve seat and thus dampen the mechanical stress and wear and tear on the valve seats. When driving in long, difficult driving conditions, damage to the valve seat is possible due to the use of unleaded petrol in engines without hardened valve seats. This damage does not occur under normal driving conditions.
Anti-knock agent for aviation fuel
In aviation gasoline tetraethyl lead is still a legal additive and is used by aircraft with a petrol engine. The AvGas 100 LL type with a lead content of 0.56 g lead per liter is predominantly used worldwide. The U.S. Environmental Protection Agency estimates that Avgas was the source of approximately 60% of lead aerosols in the United States in 2019. Since both leaded and unleaded petrol use the same pipelines in an oil refinery , an amount of 0.013 grams per liter, measured at +15 ° C, is permissible for motor petrol contaminated as a result. Aviation fuel only contains 1,2-dibromoethane as a scavenger, the molar ratio of lead to bromine is 1: 2.
Other uses
The organometallic chemical vapor deposition of tetraethyl lead and tetraisopropyl orthotitanate was used to produce phase-pure perovskite - lead titanate (PbTiO 3 ) thin films on quartz glass and platinum coated aluminum oxide substrates . Lead titanate is a technologically important ceramic that becomes ferroelectric when the Curie temperature of 447 ° C is reached and takes on a tetragonal structure . The same technique can be used to produce lead zirconate titanate films (PbZr x Ti 1 − x O 3 ) with excellent ferroelectric properties.
Further technical applications of tetraethyl lead are not known due to its toxicity. The use of tetraethyl lead has been considered in reactions in which ethyl radicals induce typical radical reactions, for example for the radical polymerization of ethene . On a laboratory scale, tetraethyl lead can be used for organometallic reactions such as ligand substitution. Ethyl arsenic dichloride can be synthesized by reacting tetraethyl lead with arsenic (III) chloride .
The use of tetraethyl lead to produce alkyl mercury compounds used as fungicides and dressings is also known. The use of tetraethyl lead in coal liquefaction through direct hydrogenation was investigated. Almost all of the coal was converted into distillable oil and gas fractions.
Environmental relevance
Environmental pollution from tetraethyl lead results from soil contamination with leaded gasoline, with tetraethyl lead itself and with scavengers such as 1,2-dibromoethane and 1,2-dichloroethane. Due to the accident of the Yugoslav cargo ship "Cavtat" in 1974 about 320 tons of tetraethyl lead and tetramethyl lead sank in 900 barrels in the Italian Adriatic Sea near Otranto . Divers from a company specializing in accidents later recovered the barrels. Tetraethyl burns in the engine to Bleioxochlorid and Bleioxobromid and other lead compounds. Around 75% of the lead is emitted into the environment, some of which is deposited on the exhaust manifold and the exhaust .
Tetraethyl lead has been the major source of lead contamination of the environment worldwide. The analysis of the lead concentration in the Greenland ice sheets showed that lead concentrations increased 200-fold after 1940 compared to the values of 800 BC. Smaller increases in concentration between the 5th century BC and the 3rd century AD can be traced back to the Roman lead processing .
It is estimated that between 1926 and 1985 in the United States over seven million tons of lead were added to gasoline as tetraalkyl lead, burned, and emitted as lead oxide particles. In New Orleans alone , around 10,000 tons of lead were deposited, mostly near busy roads. Soil contaminated with lead remains a seasonal source of lead exposure. As the soil moisture drops in summer and autumn, lead-contaminated dust is blown up and inhaled.
In some cases it has been speculated that the forest dieback is due to the toxic effects of triethylene lead ion as a breakdown product of incompletely burned leaded gasoline. However, the assumption could not be confirmed.
Emitted lead gets back into the human body from the environment through contamination of food and food intake. In 2005, for example, the lead concentrations of tested cocoa and chocolate products were 230 and 70 nanograms per gram, respectively. The contamination of the products was attributed to the emissions from the burning of tetraethyl lead, which was still used as an anti-knock agent in 2005 in cocoa-growing areas such as Nigeria . The contamination took place not only during cultivation, but in the entire processing chain of the chocolate products. High lead concentrations in products that are primarily marketed to children are particularly worrying because of the susceptibility of children to lead poisoning. The maximum permissible lead content proposed by the Codex Alimetarius Commission is only 0.1 nanograms per gram for cocoa butter .
The soil is contaminated with leaded gasoline, for example, through leaks from underground tanks . There were more than 200,000 gas stations in the United States in the 1960s, many of which have been abandoned over the years. Many of these gas stations had multiple underground tanks. Leakage is quite common with old underground tanks, with more than 400,000 releases of leaded gasoline on record in the United States , according to the Environmental Protection Agency .
When the scavengers are burned, bromomethane is also produced . Bromomethane is easily subject to photolysis in the atmosphere , releasing bromine radicals which are responsible for the depletion of stratospheric ozone . As such, it is subject to the exit requirements of the Montreal Protocol on Ozone Depleting Substances.
The incineration of chlorinated scavengers enables the formation and emission of polychlorinated dibenzodioxins and dibenzofurans . However, due to the variety and number of vehicles, their technical equipment and the difficulties in detecting them under different environmental conditions, the level of emissions is unclear. In tests under controlled conditions, various polychlorinated dibenzodioxins and dibenzofurans were found in motor oil.
toxicology

Lead is a natural component of the earth's crust, the average content is around 0.0018%. Lead compounds are often toxic even in small quantities; Lead is a cumulative neurotoxin with serious negative effects on the nervous, circulatory, reproductive, renal and digestive systems, with symptoms not appearing until a threshold value is exceeded. Its toxicity was already known and documented in pre-Christian times. The Greek doctor and poet Nikandros from Kolophon reported about colic and anemia from lead poisoning around 250 BC . Pedanios Dioskurides described the neurotoxic properties of lead in the first century AD, stating that "lead makes the mind wane".
The Romans used lead and its compounds in many ways. They used lead presses in winemaking and preserved wine with defrutum , a grape juice concentrate that was boiled down in lead vessels. They used lead-containing dishes and the aqueducts were partly lined with lead. The neurotoxic effects of the resulting lead exposure presumably contributed to the decline of the Roman Empire .
In later centuries, lead poisoning mainly affected certain occupational groups, such as painters , typesetters or employees in the ceramics industry. These occupational groups ingested lead by inhaling dust containing lead or by consuming lead-contaminated food. With the introduction of tetraethyl lead as an anti-knock agent in 1922, lead emissions reached a new level. In just over seven decades, vehicles around the world distributed several million tons of lead, especially in cities and along main roads. The British daily The Guardian called the use of tetraethyl lead in motor gasoline in 2018 "by far the largest mass poisoning experiment of all time".
Tetraethyl lead poisoning
Tetraethyl lead is highly toxic; the ingestion of a few milliliters is enough to cause severe poisoning in humans. Because of its lipophilicity , tetraethyl lead can easily cross the blood-brain barrier and accumulate in the limbic system , frontal lobe, and hippocampus, causing acute poisoning. The lethal dose for tetraethyl lead was determined in animal experiments in rats with intragastric application to be 14.18 mg / kg. Poisoning occurs, for example, through absorption through the skin , swallowing or as a result of inhalation, for example during the manufacture of tetraethyl lead, improper handling of leaded petrol or from sniffing petrol . Compared to normal lead poisoning, tetraethyl lead poisoning is usually acute and attacks the central nervous system .
The symptoms of tetraethyl lead poisoning include a drop in blood pressure and body temperature , sleep disorders , headaches , urge to move , hypersensitivity to auditory and tactile stimuli, loss of appetite , tremors , hallucinations , psychoses and severe aggressiveness. It also causes a strong urge to sneeze .
Tetraethyl lead is cleaved in the liver to form triethyl lead ion ((C 2 H 5 ) 3 Pb + ), and then further metabolized to diethyl lead ion ((C 2 H 5 ) 2 Pb 2+ ) and ionic lead (Pb 2+ ). The actual toxic species is triethyl lead ion. In animal experiments, about half of the tetraethyl lead converted to this after 24 hours. The body excretes lead in the urine as diethyl lead and in the feces as inorganic lead. Tetraethyl lead in unburned fuel can lead to poisoning through uncontrolled evaporation or improper use of the fuel.
Particle poisoning
The lead oxide and lead halide particle exposure caused by the combustion of leaded gasoline does not normally cause immediate lead poisoning. The lead emitted with vehicle exhaust gases is absorbed directly with the air we breathe or through skin dust and food. In adults, more than 90% of the inorganic lead is stored in the bones, with a half-life of around 30 years.
A reduction in lead emissions from motor vehicles thus has a significant effect on reducing the lead pollution of the population. As far as is known today, the toxic effect of lead is based on the inactivation of various enzymes. This results in an inhibition of blood pigment synthesis or direct damage to red blood cells. Anemia as well as damage to the lung cleaning function and various metabolic processes are associated with increased lead exposure. In particular, functional disorders in the central nervous system in children were observed at correspondingly high levels of lead, some of which are irreversible.
The average blood lead content of American children in 2013 was 1.2 micrograms per deciliter (µg / dl). In the years 1976 to 1980 the average value was 15 μg / dl. In 2012, the Centers for Disease Control and Prevention set a threshold value for blood lead content of 5 μg / dl, halving the intervention level that had been in effect until then. As other sources of lead contamination was in the United States in 1978 lead in paints , in 1986 for water pipes and 1995 as part of the solder for cans prohibited.
The relationship between lead blood levels and cognitive abilities was determined in preschool children. Although lead levels were below the criteria for lead poisoning, static analyzes showed that there was a correlation between increasing lead levels and decreases in general cognitive, verbal and perceptual skills.
Carcinogenicity
Studies in the 1970s denied a link between lead exposure and cancer mortality in humans. However, later research into exposure to car exhaust showed that people who lived near a busy road were at higher risk of cancer than people who lived in a low-traffic area. These results led to the suspicion that there is a connection between the occurrence of cancer and the emitted lead particles, possibly in combination with other carcinogenic substances.
More recent studies affirm an epidemiological relationship between lead exposure and cancer risk in humans, although lead alone may not be necessary and sufficient for causing cancer. Although the biochemical and molecular mechanisms of action of lead are still unclear, it is believed that lead can exacerbate the carcinogenic events involved. The International Agency for Research on Cancer has now classified lead as a possible human carcinogen (Group 2B) and its inorganic compounds as a likely human carcinogen (Group 2A).
Influence on crime rate and the intelligence coefficient
Various studies have concluded that lead exposure in childhood can lead to aggressive and criminal behavior. Studies in Chicago , Indianapolis , Minneapolis and other major US cities showed a close statistical association between lead exposure in childhood and serious bodily harm with a time lag of about 23 years. Although the findings are inconsistent, they suggest an effect of lead exposure on homicide rates. It appears that the phasing out of leaded petrol from the late 1970s is related to a decline in violent crime from the 1990s. Other studies found a statistical association between childhood lead exposure and the extent, type, and impact of violent crime in the United States with data from the Uniform Crime Reporting Program (UCR) but not data from the National Crime Victimization Survey (NCVS).
Some sociologists saw the rise in the crime rate since the early 1960s as the beginning of a reversal in the long-term decline in crime . Some crime historians suggest that this is only a small deviation from the centuries-old trend of decline, and that shifts in ideals of lifestyle explain a long-term decline in interpersonal violence.
In addition to the connection between lead-induced neurotoxicity and aggressiveness as well as crime, which has been assumed in many studies, other studies see a connection between lead exposure and intellectual deficits that manifest themselves from childhood to adolescence. For example, the concentration of lead in the soil, which in central locations in large cities can be more than 100 times the value in rural areas, and the effects on mental and behavioral deficits were assessed.
However, critics objected to the design of various epidemiological studies. They also criticized the possibility of sample distortion and the inadequate assessment of the analytical difficulties in measuring the lead exposure of the body and in measuring the intelligence quotient of a child. In addition, they judged the use of statistical analysis techniques and the scope of many studies to be insufficient.
proof
Several methods have been developed for the determination of tetraethyl lead in gasoline. The standard analysis methods are based on atomic absorption spectrometry . For this purpose, the tetraalkyl lead compounds are extracted with carbon tetrachloride , for example , and oxidized with nitric acid, the lead then being determined in the graphite tube . The US method ASTM D3237 (Standard Test Method for Lead in Gasoline by Atomic Absorption Spectroscopy) or DIN EN 237 (Liquid petroleum products - Otto fuel - Determination of low lead contents by atomic absorption spectrometry) can be used to detect small amounts of lead . The method is suitable for the determination of lead in water, air and biological material.
Another common standard process was the iodine monochloride process, which is described in DIN EN ISO 3830. By means of X-ray fluorescence analysis beside tetraethyl as scavengers such as 1,2-dibromoethane can determine, the lead concentration can be in a range of 0.02 to 0.24 wt .-%.
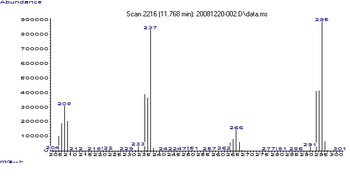
The various lead alkyls can be determined side by side by gas chromatography coupled with mass spectrometry . The mass spectrum of tetraethyl lead has 64 peaks, of which the peaks with a mass-to-charge ratio of 237 ((C 2 H 5 ) Pb), 295 ((C 2 H 5 ) 3 Pb) and 208 (Pb) the most intense.
Tetraethyl lead can also be determined by decomposition with iodine and subsequent titration with potassium chromate .
The determination by means of ethylenediaminetetraacetic acid titration after oxidation of the tetraethyl lead with sulfuric acid and nitric acid was developed as a further method . The lead is precipitated as lead (II) sulfate and then converted back into solution with ammonium tartrate. The titration is carried out using Eriochrome black T as an indicator at a pH value of 10.
In the infrared spectrum , tetraethyl lead shows a strong band at a wavenumber of 240 cm -1, a strong band that is assigned to the Pb-CC bending vibration, and two moderate bands at wavenumbers of 132 and 86 cm -1 , that of the C-Pb-C -Bending vibration can be assigned.
Atomic emission spectrometry offers a quick method without sample pretreatment for the determination of lead in gasoline . The sensitivity of the method is around 0.00025 milliliters of tetraethyl lead per liter of gasoline. For routine determinations an accuracy of 0.0025 milliliters per liter is possible.
The quantitative detection of tetraethyl lead in gasoline is possible by dissolving a sample in anhydrous ethylene glycol monoethyl ether and then reacting with hydrogen chloride , whereby the resulting lead ions can be detected directly by polarography . The detection limit is around 0.13 ml / l.
literature
- Magda Lovei: Phasing out lead from gasoline: worldwide experience and policy implications . World Bank technical paper no.397, ISBN 0-8213-4157-X .
- Dietmar Seyferth : The Rise and Fall of Tetraethyllead. Part 1. In: Organometallics . 22, 2003, pp. 2346-2357, doi: 10.1021 / om030245v .
- Dietmar Seyferth: The Rise and Fall of Tetraethyllead. Part 2. In: Organometallics. 22, 2003, pp. 5154-5178, doi: 10.1021 / om030621b .
Web links
Individual evidence
- ↑ a b c Entry on tetraethyl lead. In: Römpp Online . Georg Thieme Verlag, accessed on July 17, 2014.
- ↑ a b c d e f g h i j Entry on tetraethyl lead in the GESTIS substance database of the IFA , accessed on August 21, 2018(JavaScript required) .
- ↑ a b Datasheet Tetraethyllead from Sigma-Aldrich , accessed on April 24, 2011 ( PDF ).
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry lead alkyls in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016 . marketer can use the harmonized classification and labeling expand .
- ↑ a b Entry in the SVHC list of the European Chemicals Agency , accessed on July 16, 2014.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 78-00-2 or tetraethyl lead ), accessed on November 2, 2015.
- ↑ a b c d e f Dietmar Seyferth: The Rise and Fall of Tetraethyllead. 1. Discovery and Slow Development in European Universities, 1853-1920. In: Organometallics . 22, 2003, pp. 2346-2357, doi: 10.1021 / om030245v .
- ^ Edward Frankland, In: Liebigs Annalen der Chemie und Pharmacie , 71, 1849, p. 213.
- ^ Edward Frankland: On the isolation of the organic radicals. In: J. Chem. Soc. , 2, 1850, p. 263.
- ↑ Reinhard Seifert: The era of Gottlieb Daimler: New perspectives on the early history of the automobile and its technology . Vieweg and Teubner Research, Wiesbaden, ISBN 978-3-8348-0962-9 , p. 32.
- ↑ a b c d e f g Dietmar Seyferth: The Rise and Fall of Tetraethyllead. 2. In: Organometallics. 22, 2003, pp. 5154-5178, doi: 10.1021 / om030621b .
- ^ A b c Alan P. Loeb: Birth of the Kettering Doctrine: Fordism, Sloanism and the Discovery of Tetraethyl Lead. In: Business and Economic History , Vol. 24, No. 1, Papers presented at the forty-first annual meeting of the Business History Conference (Fall 1995), pp. 72-87.
- ↑ Patent US1639947A : Art of making metallo-organic compounds. Published April 13, 1923 , inventors: Charles A Kraus, Conrad C. Callis.
- ^ Homepage of the Ethyl Corporation .
- ^ Tom McCarthy: Auto Mania: Cars, Consumers, and the Environment. Yale University Press, New Heaven, London, 2007, ISBN 978-0-300-11038-8 , p. 49.
- ↑ Gerald Markowitz, David Rosner: Deceit and Denial: The Deadly Politics of Industrial Pollution. University of California Press, Berkeley, Los Angeles, London, 2002, ISBN 0-520-24063-4 , p. 25.
- ↑ a b c M. Mosimann, M. Breu, T. Vysusil, S. Gerber: From the tiger in the tank - The history of the blebenzins. In: GAIA . 2002, 11, pp. 203-212, doi: 10.14512 / gaia.11.3.12 ( PDF ).
- ^ A b Colleen F. Moore: Children and Pollution. Why Scientists Disagree . Oxford University Press, 2009, ISBN 978-0-19-538666-0 , p. 6.
- ↑ Deborah Blum: Looney Gas and Lead Poisoning: A Short, Sad History . In: Wired.com, May 1, 20131.
- ↑ Stanton P. Nickerson: Tetraethyl lead: A product of American research. In: Journal of Chemical Education . 31, 1954, pp. 560-571, doi: 10.1021 / ed031p560 .
- ↑ CFKettering: Thomas Midgley, jr .: An Appreciation. In: Science . 100, 1944, pp. 562-564, doi: 10.1126 / science.100.2608.562 .
- ↑ a b c Jerome O. Nriagu: The rise and fall of leaded gasoline. In: Science of the total environment . 92, 1990, pp. 13-28.
- ↑ Jacques R. Pauwels: Profits “About Everything!” American Corporations and Hitler. In: Labor / Le Travail , 51, 2003, pp. 223–249.
- ^ Charles G. Moseley: Eugene Houdry, catalytic cracking and World War II aviation gasoline. In: Journal of Chemical Education , 61, 1984, p. 655.
- ↑ Joseph Borkin: The unholy alliance of the IG colors. A community of interests in the Third Reich. Campus, Frankfurt am Main 1990, ISBN 3-593-34251-0 , p. 76.
- ^ Rainer Karlsch , Raymond G. Stokes: Factor oil. The mineral oil industry in Germany 1859–1974. CH Beck, Munich, 2003, ISBN 3-406-50276-8 , p. 187.
- ↑ Wolfgang Birkenfeld : The synthetic fuel 1933-1945. A contribution to the National Socialist economic and armaments policy. Göttingen, Berlin, Frankfurt: Musterschmidt 1964, p. 64.
- ^ Stacy Cagle Davis, Robert Gary Boundy: Transportation Energy Data Book . Edition 38. No. ORNL / TM-2019/1333. Oak Ridge National Lab. (ORNL), Oak Ridge, Tennessee 37831-6073 (United States), 2020. US Department of Energy, Contract No. DE-AC05-00OR22725.
- ↑ a b Printed matter 11/4023 of February 17th, 1989, answer of the Federal Government to the small question of the MPs Ms. Garbe, Brauer, Ms. Rock, Weiss (Munich) and the parliamentary group DIE GRÜNEN: Poisons in petrol (I) , printed matter 11/3843 .
- ↑ Mike McPhate: When LA smog was so bad people suspected a gas attack. In: California Sun , July 9, 2018.
- ^ A b c d e Herbert L. Needleman : The Removal of Lead from Gasoline: Historical and Personal Reflections. In: Environmental Research . 84, 2000, pp. 20-35, doi: 10.1006 / enrs.2000.4069 .
- ↑ a b Jerome O. Nriagu: Clair Patterson and Robert Kehoe's paradigm of "Show Me the Data" on Environmental Lead Poisoning. In: Environmental Research. 78, 1998, pp. 71-78, doi: 10.1006 / enrs.1997.3808 .
- ^ William Kovarik: Ethyl-leaded Gasoline: How a Classic Occupational Disease Became an International Public Health Disaster. In: International Journal of Occupational and Environmental Health . 11, 2013, pp. 384-397, doi: 10.1179 / oeh.2005.11.4.384 .
- ↑ Magda Lovei: Phasing out lead from gasoline: worldwide experience and policy implications . World Bank technical paper no.397, ISBN 0-8213-4157-X , p. 7.
- ↑ a b c Text of the Gasoline Lead Act
- ↑ The first anti-knocking agent factory. In: Chemie Ingenieur Technik , 38, 8, 1965, p. 692.
- ↑ Fritz Kalberlah, Markus Schwarz, Dirk Bunke, Roland Augustin, Reinhard Oppl: Carcinogens, mutagenic, toxic to reproduction (CMR) and other problematic substances in products Identification of relevant substances and products, verification by measurements, need for regulation in chemicals law. 18/2011, on behalf of the Federal Environment Agency .
- ^ United Nations Environmental Program: The Lead Campaign.
- ^ Ian Johnston: UK company sells lead to last place on Earth where leaded petrol is legal. In: The Independent , August 22, 2017.
- ↑ Viv Bernstein: Nascar Plans to Switch to Unleaded Fuel in '08. In: The New York Times , January 20, 2006.
- ↑ a b c R. Kessler: Sunset for leaded aviation gasoline? In: Environmental health perspectives . Volume 121, number 2, February 2013, pp. A54-a57, doi: 10.1289 / ehp.121-a54 , PMID 23380048 , PMC 3569701 (free full text).
- ↑ Gmelin Handbook of Inorganic Chemistry , Organo Lead Compounds, Part 2, Springer, Berlin, Heidelberg, 8th Edition, 1990, ISBN 978-3-662-10293-0 , p. 20.
- ↑ a b c d Friedrich Asinger : Chemistry and technology of paraffin hydrocarbons . Akademie Verlag, 1956, pp. 241–243.
- ↑ Patent USUS1622228A : Process of making organic lead compounds. Published on May 19, 1923 , inventor Thomas Midgley Jr ..
- ↑ Patent USUS2635106A : Process for making tetraethyl lead. Published October 25, 1951 , Inventors: Shapiro Hymin, Earl G. De Witt.
- ^ A b Robert W. Leeper, Lawrence Summers, Henry Gilman: Organolead Compounds. In: Chemical Reviews . 54, 1954, pp. 101-167, doi: 10.1021 / cr60167a004 .
- ^ Klaus Weissermel , Hans-Jürgen Arpe : Industrial Organic Chemistry: Important Raw Materials and Intermediates . Wiley-VCH Verlag, Weinheim, 2003, ISBN 3-527-30578-5 , p. 198.
- ^ C. Janiak, TM Klapötke, H.-J. Meyer, E. Riedel: Modern inorganic chemistry . 2nd edition, de Gruyter, Berlin, 2004, ISBN 978-3-11-044160-4 , pp. 656-658.
- ↑ Christoph Elschenbroich: Organometallchemie , 3rd edition, Teubner, Stuttgart, 1993, ISBN 978-3-519-33501-6 , pp. 171-178.
- ↑ George A. Hay: Facilitating Practices: The Ethyl Case (1984) . In: John E. Kwoka; Lawrence J. White: The antitrust revolution: economics, competition, and policy. Oxford University Press, New York, 1999, ISBN 978-0-19-512015-8 , pp. 182-201.
- ↑ Ionel Haiduc, Jerry J. Zuckerman: Basic Organometallic Chemistry . Walter de Gruyter, Berlin, New York, 1985, ISBN 3-11-007184-3 , pp. 174-176.
- ^ A b c A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1028-1029.
- ↑ Frederick W. Frey, Shirl E. Cook: The Synthesis of Tetraethyllead by reaction of tetravalent Lead Salts with triethylaluminum and Other Metal ethyl compounds. In: Journal of the American Chemical Society . 82, 1960, pp. 530-533, doi: 10.1021 / ja01488a008 .
- ^ R. Galli: The formation of tetraethyl lead by electrochemical reduction of ethyl bromide. In: Journal of Electroanalytical Chemistry and Interfacial Electrochemistry . 22, 1969, pp. 75-84, doi: 10.1016 / S0022-0728 (69) 80148-8 .
- ^ R. Duffy, J. Feeney, AK Holliday: 219. Trialkylplumbanes. Part II. Some properties of trimethyl- and triethyl-plumbane. In: Journal of the Chemical Society . 1962, pp. 1144-1147, doi: 10.1039 / JR9620001144 .
- ^ A b L. AK Staveley, HP Paget, BB Goalby, JB Warren: 470. Polymorphism in tetraethyl-tin and -lead. In: Journal of the Chemical Society. 1950, pp. 2290-2301, doi: 10.1039 / JR9500002290 .
- ↑ Ionel Haiduc, Jerry J. Zuckerman: Basic Organometallic Chemistry . Walter de Gruyter, Berlin, New York, 1985, ISBN 3-11-007184-3 , p. 3.
- ↑ WP Neumann, K. Kühlein: Recent Developments in the Chemistry of Organic Lead: Preparations and reaction of compounds with Pb-C, Pb-H, Pb-Pb and N-O bond. In: FGA Stone, R. West: Advances in Organometallic Chemistry , Vol. 7, Academic Press, New York, London, 1968, ISBN 978-0-12-031107-1 , pp. 242-303.
- ↑ Gregory P. Smith, Roger Patrick: Pyrolysis studies of main group metal-alkyl bond dissociation energies: VLPP of GeMe 4 , SbEt 3 , PbEt 4 , and PEt 3 . In: International Journal of Chemical Kinetics . 15, 1983, pp. 167-185, doi: 10.1002 / kin.550150207 .
- ^ Fritz Paneth, Karl Herzfeld: About free methyl and free ethyl . In: Journal of Electrochemistry and Applied Physical Chemistry , 37, pp. 577-582 (1931). doi: 10.1002 / bbpc.19310370834
- ^ Rolf Gelius: Reactions of organic clothing derivatives with sulfur compounds. III. The implementation of tetraethyl and tetramethyl lead with sulfur dioxide. In: Journal of Inorganic and General Chemistry . 349, 1967, pp. 22-32, doi: 10.1002 / zaac.19673490104 .
- ↑ Gary A. Downing, Christopher S. Frampton, James H. Gall, David D. MacNicol: Octakis (3,4-dimethylphenylthio) naphthalene: A Designed Spider Host of Unparalled Versatility. In: Angewandte Chemie International Edition in English . 35, 1996, pp. 1547-1549, doi: 10.1002 / anie.199615471 .
- ↑ Jürgen Warnatz, Ulrich Maas, Robert W. Dibble: Engine knock . In: combustion . Springer, Berlin, Heidelberg, 2001, ISBN 978-3-642-62658-6 , pp. 247-258.
- ↑ Fred Schäfer, Richard van Basshuysen (ed.): Handbook Internal Combustion Engine, Fundamentals Components Systems Perspectives , 8th Edition, Springer, Wiesbaden 2017, ISBN 978-3-658-10901-1 , p. 729
- ↑ Jürgen Wolfrum (Ed.): Gas phase chemical reaction systems: experiments and models 100 years after Max Bodenstein . Springer, Berlin, Heidelberg, New York, 1995, ISBN 978-3-642-80301-7 , p. 285.
- ^ R. Perry, C. DiPerna, D. Heath: Tetramethyl lead an Antiknock for better Road Performance . SAE Technical Paper 600141, 1960, doi: 10.4271 / 600141 .
- ↑ Christian Jentsch: Unleaded petrol. In: Chemistry in Our Time . 20, 1986, pp. 105-110, doi: 10.1002 / ciuz.19860200402 .
- ↑ a b J. Riggs: Mixed lead alkyls; volatile and selective antiknocks . 6th World Petroleum Congress, June 19-26, Frankfurt am Main, Germany, WPC-10506, 1963.
- ^ VM Thomas, JA Bedford, RJ Cicerone: Bromine emissions from leaded gasoline. In: Geophysical Research Letters . 24.11, 1997, pp. 1371-1374, doi: 10.1029 / 97GL01243 .
- ^ JE Brown, WG Lovell: A New Manganese Antiknock. In: Industrial & Engineering Chemistry . 50, 1958, pp. 1547-1550, doi: 10.1021 / ie50586a035 .
- ↑ Alexandre Joly, Jean Lambert, Claude Gagnon, Greg Kennedy, Donna Mergler, Ariane Adam-Poupart, Joseph Zayed: Reduced Atmospheric Manganese in Montreal Following Removal of Methylcyclopentadienyl Manganese Tricarbonyl (MMT). In: Water, Air & Soil Pollution . 219, 2011, pp. 263-270, doi: 10.1007 / s11270-010-0704-6 .
- ^ VM Thomas: The Elimination of Lead in Gasoline. In: Annual Review of Energy and the Environment . 20, 1995, pp. 301-324, doi: 10.1146 / annurev.eg.20.110195.001505 .
- ^ A b Howard W. Mielke, Christopher R. Gonzales, Eric T. Powell: Curtailing Lead Aerosols: Effects of Primary Prevention on Declining Soil Lead and Children's Blood Lead in Metropolitan New Orleans. In: International Journal of Environmental Research and Public Health . 16, 2019, p. 2068, doi: 10.3390 / ijerph16122068 .
- ↑ SL Swartz, DA Seifert, GT Noel, TR Shrout: Characterization of MOCVD PbTiO 3 thin films. In: Ferroelectrics . 93, 1989, pp. 37-43, doi: 10.1080 / 00150198908017318 .
- ↑ M. De Keijser, GJM Dormans, PJ Van Veldhoven, PK Larsen: High quality lead zirconate titanate films grown by organometallic chemical vapor deposition. In: Integrated Ferroelectrics . 3, 2006, pp. 131-137, doi: 10.1080 / 10584589308216707 .
- ↑ PL Kooijman, WL Ghijsen: Investigations Concerning polymerization of propene and ethene induced by tetraethyl lead. In: Recueil des Travaux Chimiques des Pays-Bas . 66, 1947, pp. 247-256, doi: 10.1002 / recl.19470660411 .
- ^ MS Kharasch, Elwood V. Jensen, Sidney Weinhouse: Alkylation Reactions of Tetraethyllead. A New Synthesis of Ethyldichloroarsine and related Compounds. In: The Journal of Organic Chemistry . 14, 1949, pp. 429-432, doi: 10.1021 / jo01155a014 .
- ↑ George Calingaert, Harold A. Beatty, Harold Soroos: The Redistribution Reaction. V. R 4 Pb Compounds. In: Journal of the American Chemical Society. 62, 1940, pp. 1099-1104, doi: 10.1021 / ja01862a030 .
- ↑ Moetaz I. Attalla, Robinson A. Quezada, Anthony M. Vassallo, Michael A. Wilson: tetraethyl lead as a coal liquefaction promoter. In: Fuel . 71, 1992, pp. 401-407, doi: 10.1016 / 0016-2361 (92) 90029-N .
- ↑ Ronald W. Falta, N. Bulsara, JK Henderson, RA Mayer: Leaded-gasoline additives still contaminate groundwater. In: Environ Sci. Technol. 39, 2005, pp. 378A-384A, PMID 16201608 ; doi: 10.1021 / es053352k .
- ↑ Ronald W. Falta: The Potential for Ground Water Contamination by the Gasoline Lead Scavengers Ethylene Dibromide and 1,2-Dichloroethane. In: Ground Water Monitoring & Remediation . 24, 2004, pp. 76-87, doi: 10.1111 / j.1745-6592.2004.tb01294.x .
- ↑ Jacques Cousteau , Susan Schiefelbein: Man, the orchid and the octopus: My life for the exploration and preservation of our environment. Campus Verlag, Frankfurt, New York, 2008, ISBN 978-3-593-38564-8 , pp. 135-137.
- ↑ M. Murozumi, Tsaihwa J Chow, C. Patterson: Chemical concentrations of pollutant lead aerosols, terrestrial dusts and sea salts in Greenland and Antarctic snow strata. In: Geochimica et Cosmochimica Acta . 33, 1969, pp. 1247-1294, doi: 10.1016 / 0016-7037 (69) 90045-3 .
- ↑ H. Faulstich, Chr. Stournaras: Does triethyl lead cause our forest damage? In: Naturwiss. Rundsch. , 37, 1984, pp. 398-401.
- ^ A b Charley W. Rankin, Jerome O. Nriagu, Jugdeep K. Aggarwal, Toyin A. Arowolo, Kola Adebayo, A. Russell Flegal: Lead Contamination in Cocoa and Cocoa Products: Isotopic Evidence of Global Contamination. In: Environmental Health Perspectives . 113, 2005, pp. 1344-1348, doi: 10.1289 / ehp.8009 .
- ↑ Gil Oudijk: The Use of Alkyl Leads in Gasoline Age-Dating Investigations: New Insights, Common Investigative Techniques, Limitations, and Recommended Practices. In: Environmental Claims Journal . 19, 2007, pp. 68-87, doi: 10.1080 / 10406020601158329 .
- ↑ B. Ramacher, J. Rudolph, R. Koppmann: Hydrocarbon measurements during tropospheric ozone depletion events: Evidence for halogen atom chemistry. In: Journal of Geophysical Research : Atmospheres. 104, 1999, pp. 3633-3653, doi: 10.1029 / 1998JD100061 .
- ^ R. Smit, K. Zeise, A. Caffin, P. Anyon: Dioxins Emissions from Motor Vehicles in Australia. In: National Dioxins Program Technical Report No. 2, P , 2004, Department of the Environment and Heritage, Canberra, ISBN 0-642-54994-X .
- ↑ Stellan Marklund, Christoffer Rappe, Mats Tysklind, Karl-Erik Egebäck: Identification of polychlorinated dibenzofurans and dioxins in exhausts from cars run on leaded gasoline. In: Chemosphere . 16, 1987, pp. 29-36, doi: 10.1016 / 0045-6535 (87) 90105-6 .
- ↑ Hans Breuer: General and inorganic chemistry. (= dtv-Atlas Chemie. Volume 1). 9th edition. dtv, Munich 2000, ISBN 3-423-03217-0 , p. 151.
- ^ Herbert L. Needleman, David Gee: Lead in petrol makes the mind "give way". Late lessons from early warnings: science, precaution, innovation . Copenhagen, European Environment Agency, 2013.
- ^ A b c Herbert L. Needleman: History of lead poisoning in the world . Lecture at the International Conference on lead Poisoning Prevention and Treatment , Bangalore, 1999.
- ↑ Geoffrey Lean: Lead is even deadlier than we feared as the full extent of its toxic effects are revealed . In: The Guardian . March 17, 2018, accessed March 1, 2020.
- ↑ T. Schroeder, DD Avery, HA Cross: The LD50 value of tetraethyl lead. In: Experientia . 28, 1972, pp. 425-426, doi: 10.1007 / BF02008318 .
- ^ AD Beattie, MR Moore, A. Goldberg: Tetraethyllead-Poisoning. In: The Lancet . 300, 1972, pp. 12-15, doi: 10.1016 / S0140-6736 (72) 91276-7 .
- ^ RL Boeckx, B. Postl, FJ Coodin: Gasoline sniffing and tetraethyl lead poisoning in children. In: Pediatrics . 60.2, 1977, pp. 140-145, PMID 887326 .
- ↑ E. Oberdisse: Pharmakologie und Toxikologie , Springer, Berlin, Heidelberg, New York, 1999, ISBN 978-3-642-98031-2 , pp. 716-717.
- ↑ Z. Turlakiewicz, J. Chmielnicka: Diethyllead as a specific indicator of occupational exposure to tetraethyllead. In: British Journal of Industrial Medicine . Volume 42, number 10, October 1985, pp. 682-685, doi: 10.1136 / oem.42.10.682 , PMID 4041386 , PMC 1007558 (free full text).
- ↑ Regine Gihr, Gerd Rippen : Derivation of a preliminary insignificance threshold value for methyl and ethyl lead compounds (GFS lead alkyls). Hessian State Office for Geology and Environment, October 21, 2011, 31 pages.
- ^ Rainer Braun, Günter Fred Fuhrmann, Wolfgang Legrum, Christian Steffen: Special toxicology for chemists: A selection of toxic substances. Teubner Verlag, Stuttgart, Leipzig, 1999, ISBN 978-3-519-03538-1 , pp. 34-37.
- ^ Ruth A. Lawrence, Christof Schaefer: Industrial chemicals and environmental contaminants. In: Christof Schaefer, Paul Peters, Richard K. Miller: Drugs During Pregnancy and Lactation. Treatment Options and Risk Assessment. Academic Press, 2015, ISBN 978-0-12-408078-2 , pp. 863-892.
- ^ Food and Drug Administration, Department of Health and Human Services: Lead-Soldered Food Cans . In: Federal Register , Volume 60, Number 123 (June 27, 1995), pp. 33106-33109. FR Doc No: 95-15593.
- ^ Joseph Perino, Claire B. Ernhart, The Relation of Subclinical Lead Level to Cognitive and Sensorimotor Impairment in Black Preschoolers. In: Journal of Learning Disabilities . 7, 2016, pp. 616-620, doi: 10.1177 / 002221947400701006 .
- ↑ Michael R. Moore, Peter A. Meredith: The carcinogenicity of lead. In: Archives of Toxicology . 42, 1978, pp. 87-94, doi: 10.1007 / BF00316488 .
- ^ W. Blumer, Th. Reich: Leaded gasoline - A cause of cancer. In: Environment International . 3, 1980, pp. 465-471, doi: 10.1016 / 0160-4120 (80) 90154-3 .
- ↑ E. Silbergeld: Facilitative mechanisms of lead as a carcinogen. In: Mutation Research / Fundamental and Molecular Mechanisms of Mutagenesis . 533, 2003, pp. 121-133, doi: 10.1016 / j.mrfmmm.2003.07.010 .
- ↑ Julia García-Lestóna, Josefina Méndez, Eduardo Pásaroa, Blanca Laffona: Genotoxic effects of lead: An updated review . In: Environment International . 36, 6, 2010, pp. 623-636, doi: 10.1016 / j.envint.2010.04.011 .
- ↑ Long-term homicides trend in Europe.
- ^ Howard W. Mielke, Bruce Blake, Sarah Burroughs, Nancy Hassinger: Urban lead levels in Minneapolis: The case of the Hmong children. In: Environmental Research. 34, 1984, pp. 64-76, doi: 10.1016 / 0013-9351 (84) 90076-8 .
- ↑ Jessica Wolpaw Reyes: Environmental Policy as Social Policy? The Impact of Childhood Lead Exposure on Crime. In: The BE Journal of Economic Analysis & Policy . 7, 2007, doi: 10.2202 / 1935-1682.1796 .
- ↑ Janet L. Lauritsen, Maribeth L. Rezey, Karen Heimer: When Choice of Data Matters: Analyzes of US Crime Trends, 1973–2012. In: Journal of Quantitative Criminology . 32, 2016, pp. 335-355, doi: 10.1007 / s10940-015-9277-2 .
- ^ Helmut Thome: Explaining Long Term Trends in Violent Crime. In: Crime, Histoire & Sociétés . 5, 2001, pp. 69-86, doi: 10.4000 / chs.738 .
- ↑ Manuel Eisner: Modernity strikes back? A historical perspective on the latest increase in interpersonal violence (1960–1990). In: International Journal of Conflict and Violence , 2008, pp. 288-316.
- ↑ Howard W. Mielke, Sammy Zahran: The urban rise and fall of air lead (Pb) and the latent surge and retreat of societal violence. In: Environment International . 43, 2012, pp. 48-55, doi: 10.1016 / j.envint.2012.03.005 .
- ↑ Stuart J. Pocock, Deborah Ashby: Environmental Lead and Children's Intelligence: A Review of Recent Epidemiological Studies. In: The Statistician . 34, 1985, pp. 31-44, doi: 10.2307 / 2987502 .
- ↑ K. -H. Diehl, A. Rosopulo, W. Kreuzer: Determination of lead from tetraalkyl lead compounds with the aid of AAS. In: Fresenius' magazine for analytical chemistry . 314, 1983, pp. 755-757, doi: 10.1007 / BF00467109 .
- ↑ Horst Czichos: The engineering knowledge . Springer, Berlin, Heidelberg, 2012, ISBN 978-3-642-22849-0 , p. 79.
- ↑ FW Lamb, LM Niebylski, EW Kiefer: Determination of Tetraethyllead in Gasoline by X-Ray Fluorescence. In: Analytical Chemistry . 27, 1955, pp. 129-132, doi: 10.1021 / ac60097a042 .
- ^ HE Howard, WC Ferguson, LR Snyder: Determination of tetramethyllead and tetraethyllead in gasoline by mass spectrometry. In: Analytical Chemistry. 32, 1960, pp. 1814-1815, doi: 10.1021 / ac50153a033 .
- ^ HJ Dawson: Determination of Methyl-Ethyl Lead Alkyls in Gasoline by Gas Chromatography with an Electron Capture Detector. In: Analytical Chemistry. 35, 1963, pp. 542-545, doi: 10.1021 / ac60197a001 .
- ↑ Entry on tetraethyl lead (Henry's Law data). In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed March 1, 2020.
- ^ BE Gordon, RA Burdett: Microdetermination of Tetraethyllead in Gasoline. In: Analytical Chemistry. 19, 1947, pp. 137-140, doi: 10.1021 / ac60002a021 .
- ↑ M. Brandt, RH Vanden Berg: Determination of Tetraethyllead in Gasoline by Titration with (Ethylenedinitrilo) tetraacetate. In: Analytical Chemistry. 31, 1959, p. 1921, doi: 10.1021 / ac60155a001 .
- ^ BJ Aylett: Organometallic Compounds: The Main Group Elements. Chapman and Hall, London, 1979, ISBN 978-94-009-5731-2 , pp. 280-286.
- ^ P. Gilbert: Determination of Tetraethyllead in Gasoline by Flame Photometry. In: Symposium on Flame Photometry , West Conshohocken, PA: ASTM International, 1952, pp. 77-91, doi: 10.1520 / STP39248S .
- ^ KA Hansen, TD Parks, Louis. Lykken: Rapid Polarographic Determination of Tetraethyllead in Gasoline. In: Analytical Chemistry. 22, 1950, pp. 1232-1233, doi: 10.1021 / ac60046a003 .