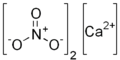List of inorganic compounds
The following list shows a selection of inorganic compounds . The list does not claim to be complete. In the first part the salts of the elements are listed according to increasing molar mass of the most important two-element compounds and oxygen acids.
The table below lists colloquial names (common names ) with the corresponding systematic names ( IUPAC names ) of known inorganic compounds alphabetically.
Inorganic compounds made up of two elements
The following overview gives an overview of inorganic compounds, which consist of two different elements.
Hydrides
Salt-like hydrides contain negatively charged hydrogen ions , the hydride ions.
| Element ( cation ) | Oxidation state | Hydride |
|---|---|---|
| lithium | +1 | Lithium hydride (LiH) |
| beryllium | +2 | Beryllium hydride (BeH 2 ) |
| sodium | +1 | Sodium hydride (NaH) |
| magnesium | +2 | Magnesium hydride (MgH 2 ) |
| aluminum | +3 | Aluminum hydride (AlH 3 ) |
| potassium | +1 | Potassium hydride (KH) |
| Calcium | +2 | Calcium hydride (CaH 2 ) |
| titanium | +2 | Titanium dihydride (TiH 2 ) |
| copper | +1 | Copper hydride (CuH) |
| zinc | +2 | Zinc (II) hydride (ZnH 2 ) |
| Rubidium | +1 | Rubidium hydride (RbH) |
| strontium | +2 | Strontium hydride (SrH 2 ) |
| yttrium | +3 | Yttrium hydride (YH 3 ) |
| Zirconium | +2 | Zirconium hydride (ZrH 2 ) |
| cadmium | +2 | Cadmium hydride (CdH 2 ) |
| Cesium | +1 | Cesium hydride (CsH) |
| barium | +2 | Barium hydride (BaH 2 ) |
| Europium | +2 | Europium (II) hydride (EuH 2 ) |
| mercury | +1 | Mercury (I) hydride (HgH) |
| +2 | Mercury (II) hydride (HgH 2 ) | |
| Thallium | +3 | Thallium hydride (TlH 3 ) |
| uranium | +3 | Uranium (III) hydride (UH 3 ) |
| plutonium | +2 | Plutonium (II) hydride (PuH 2 ) |
| Americium | +3 | Americium (III) hydride (AmH 3 ) |
Halides
Many known salts contain halogens , i.e. fluorine , chlorine , bromine or iodine . They are called fluorides , chlorides , bromides , iodides or generally halides and are derived from the hydrogen halides and their acids , the hydrohalic acids.
Chalcogenides
The salts, which contain elements of the 6th main group of the periodic table , the chalcogens , are called oxides , sulfides , selenides , tellurides or generally chalcogenides .
Nitrides, phosphides, arsenides and antimonides
The salts, which contain elements of the 5th main group of the periodic table , are the nitrides , phosphides , arsenides and antimonides .
Borides, carbides, silicides, aurides and polonides
Salts of oxygen acids
The salts of the oxo acids have an anion that consists of oxygen and at least one other element .
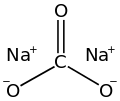
Carbonates, nitrates, phosphates and sulfates
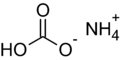
Hydrogen carbonates, hydrogen phosphates, dihydrogen phosphates and hydrogen sulfates
| Element ( cation ) | Oxidation state | Bicarbonate | Hydrogen phosphate | Dihydrogen phosphate | Hydrogen sulfate |
|---|---|---|---|---|---|
| sodium | +1 | Sodium bicarbonate (NaHCO 3 ) | Disodium hydrogen phosphate (Na 2 HPO 4 ) |
Sodium dihydrogen phosphate (NaH 2 PO 4 )
Disodium dihydrogen diphosphate (Na 2 H 2 P 2 O 7 ) |
Sodium hydrogen sulfate (NaHSO 4 ) |
| magnesium | +2 | Magnesium hydrogen carbonate (Mg (HCO 3 ) 2 ) | Magnesium hydrogen phosphate (MgHPO 4 ) | Magnesium dihydrogen phosphate (Mg (H 2 PO 4 ) 2 ) | |
| potassium | +1 | Potassium hydrogen carbonate (KHCO 3 ) | Dipotassium hydrogen phosphate (K 2 HPO 4 ) |
Potassium dihydrogen phosphate (KH 2 PO 4 )
Dipotassium dihydrogen diphosphate (K 2 H 2 P 2 O 7 ) |
Potassium hydrogen sulfate (KHSO 4 ) |
| Calcium | +2 | Calcium hydrogen carbonate (Ca (HCO 3 ) 2 ) | Calcium hydrogen phosphate (CaHPO 4 ) | Calcium dihydrogen phosphate (Ca (H 2 PO 4 ) 2 ) |
Nitrites, sulfites, hydrogen sulfites, thiosulfates and persulfates
Chlorates, bromates and iodates
Perchlorates, perbromates and periodates
| Element ( cation ) | Oxidation state | Perchlorate | Perbromate | Periodate |
|---|---|---|---|---|
| lithium | +1 | Lithium perchlorate (LiClO 4 ) | Lithium perbromate (LiBrO 4 ) | Lithium periodate (LiIO 4 ) |
| beryllium | +2 | Beryllium perchlorate (Be (ClO 4 ) 2 ) | Beryllium Perbromate (Be (BrO 4 ) 2 ) | Beryllium periodate (Be (IO 4 ) 2 ) |
| sodium | +1 | Sodium perchlorate (NaClO 4 ) | Sodium perbromate (NaBrO 4 ) | Sodium periodate (NaIO 4 ) |
| magnesium | +2 | Magnesium perchlorate (Mg (ClO 4 ) 2 ) | Magnesium Perbromate (Mg (BrO 4 ) 2 ) | Magnesium periodate (Mg (IO 4 ) 2 ) |
| potassium | +1 | Potassium perchlorate (KClO 4 ) | Potassium perbromate (KBrO 4 ) | Potassium Periodate (KIO 4 ) |
| Calcium | +2 | Calcium perchlorate (Ca (ClO 4 ) 2 ) | Calcium Perbromate (Ca (BrO 4 ) 2 ) | Calcium periodate (Ca (IO 4 ) 2 ) |
| Rubidium | +1 | Rubidium Perchlorate (RbClO 4 ) | Rubidium Perbromate (RbBrO 4 ) | Rubidium Periodate (RbIO 4 ) |
| strontium | +2 | Strontium perchlorate (Sr (ClO 4 ) 2 ) | Strontium Perbromate (Sr (BrO 4 ) 2 ) | Strontium Periodate (Sr (IO 4 ) 2 ) |
| silver | +1 | Silver perchlorate (AgClO 4 ) | Silver perbromate (AgBrO 4 ) | Silver period (AgIO 4 ) |
| Cesium | +1 | Cesium perchlorate (CsClO 4 ) | Cesium perbromate (CsBrO 4 ) | Cesium Periodate (CsIO 4 ) |
| barium | +2 | Barium perchlorate (Ba (ClO 4 ) 2 ) | Barium perbromate (Ba (BrO 4 ) 2 ) | Barium Periodate (Ba (IO 4 ) 2 ) |
Hypochlorites, hypobromites and hypoiodites
| Element ( cation ) | Oxidation state | Hypochlorite | Hypobromite | Hypoiodite |
|---|---|---|---|---|
| lithium | +1 | Lithium hypochlorite (LiClO) | Lithium hypobromite (LiBrO) | Lithium hypoiodite (LiIO) |
| beryllium | +2 | Beryllium hypochlorite (Be (ClO) 2 ) | Beryllium hypobromite (Be (BrO) 2 ) | Beryllium hypoiodite (Be (IO) 2 ) |
| sodium | +1 | Sodium hypochlorite (NaClO) | Sodium hypobromite (NaBrO) | Sodium hypoiodite (NaIO) |
| magnesium | +2 | Magnesium hypochlorite (Mg (ClO) 2 ) | Magnesium hypobromite (Mg (BrO) 2 ) | Magnesium hypoiodite (Mg (IO) 2 ) |
| potassium | +1 | Potassium hypochlorite (KClO) | Potassium hypobromite (KBrO) | Potassium hypoiodite (KIO) |
| Calcium | +2 | Calcium hypochlorite (Ca (ClO) 2 ) | Calcium hypobromite (Ca (BrO) 2 ) | Calcium hypoiodite (Ca (IO) 2 ) |
Borates, aluminates and silicates
Titanates, vanadates, chromates, manganates and permanganates
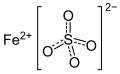
Ferrates, arsenates, arsenites, selenates and selenites
| Element ( cation ) | Oxidation state | Ferrate | Arsenate | Arsenite | Selenate | Selenite |
|---|---|---|---|---|---|---|
| lithium | +1 | Lithium ferrate (Li 2 FeO 4 ) | Lithium arsenate (Li 3 AsO 4 ) | Lithium arsenite (LiAsO 2 ) | Lithium selenate (Li 2 SeO 4 ) | Lithium selenite (Li 2 SeO 3 ) |
| beryllium | +2 | Beryllium Ferrate (BeFeO 4 ) | Beryllium arsenate (Be 3 (AsO 4 ) 2 ) | Beryllium Selenate (BeSeO 4 ) | Beryllium Selenite (BeSeO 3 ) | |
| sodium | +1 | Sodium Ferrate (Na 2 FeO 4 ) |
Sodium arsenate (Na 3 AsO 4 )
Disodium hydrogen arsenate (Na 2 HAsO 4 ) Sodium dihydrogen arsenate (NaH 2 AsO 4 ) |
Sodium arsenite (NaAsO 2 ) | Sodium selenate (Na 2 SeO 4 ) | Sodium selenite (Na 2 SeO 3 ) |
| magnesium | +2 | Magnesium Ferrate (MgFeO 4 ) | Magnesium arsenate (Mg 3 (AsO 4 ) 2 ) | Magnesium Selenate (MgSeO 4 ) | Magnesium Selenite (MgSeO 3 ) | |
| potassium | +1 | Potassium ferrate (K 2 FeO 4 ) | Potassium arsenate (K 3 AsO 4 ) | Potassium Metaarsenite (KAsO 2 ) | Potassium selenate (K 2 SeO 4 ) | Potassium selenite (K 2 SeO 3 ) |
| Calcium | +2 | Calcium Ferrate (CaFeO 4 ) | Calcium arsenate (Ca 3 (AsO 4 ) 2 ) | Calcium Selenate (CaSeO 4 ) | Calcium Selenite (CaSeO 3 ) | |
| copper | +2 | Copper (II) arsenate (Cu 3 (AsO 4 ) 2 ) | Copper (II) arsenite (CuHAsO 3 ) | Kupferselenit (CuSeO 3 ) | ||
| Rubidium | +1 | Rubidium Ferrate (Rb 2 FeO 4 ) | Rubidium arsenate (Rb 3 AsO 4 ) | Rubidium Arsenite (RbAsO 2 ) | Rubidium Selenate (Rb 2 SeO 4 ) | Rubidium Selenite (Rb 2 SeO 3 ) |
| strontium | +2 | Strontium Ferrate (SrFeO 4 ) | Strontium Arsenate (Sr 3 (AsO 4 ) 2 ) | Strontium Selenate (SrSeO 4 ) | Strontium Selenite (SrSeO 3 ) | |
| silver | +1 | Silver arsenate (Ag 3 AsO 4 ) | Silver selenite (Ag 2 SeO 3 ) | |||
| Cesium | +1 | Cesium ferrate (Cs 2 FeO 4 ) | Cesium arsenate (Cs 3 AsO 4 ) | Cesium arsenite (CsAsO 2 ) | Cesium selenate (Cs 2 SeO 4 ) | Cesium selenite (Cs 2 SeO 3 ) |
| barium | +2 | Barium ferrate (BaFeO 4 ) | Barium arsenate (Ba 3 (AsO 4 ) 2 ) | Barium Selenate (BaSeO 4 ) | Barium selenite (BaSeO 3 ) | |
| gold | +3 | Gold (III) selenate (Au 2 (SeO 4 ) 3 ) | ||||
| lead | +2 | Lead (II) arsenate (Pb 3 (AsO 4 ) 2 ) | Lead (II) selenate (PbSeO 4 ) |
Molybdates, Tellurites, Tellurates, Tantalates, Perxenates and Wolframates
| Element ( cation ) | Oxidation state | Molybdate | Tellurite | Tellurate | Tantalate | Perxenat | Tungstate |
|---|---|---|---|---|---|---|---|
| lithium | +1 | Lithium molybdate (Li 2 MoO 4 ) | Lithium tellurate (Li 2 TeO 4 ) | Lithium tantalate (LiTaO 3 ) | Lithium tungstate (Li 2 WO 4 ) | ||
| beryllium | +2 | Beryllium molybdate (BeMoO 4 ) | Beryllium tellurate (BaTeO 4 ) | Beryllium tungstate (BaWO 4 ) | |||
| sodium | +1 | Sodium molybdate (Na 2 MoO 4 ) | Sodium tellurite (Na 2 TeO 3 ) | Sodium tellurate (Na 2 TeO 4 ) | Sodium tantalate (NaTaO 3 ) | Sodium perxenate (Na 4 XeO 6 ) | Sodium tungstate (Na 2 WO 4 ) |
| magnesium | +2 | Magnesium molybdate (MgMoO 4 ) | Magnesium tellurate (MgTeO 4 ) | Magnesium tungstate (MgWO 4 ) | |||
| potassium | +1 | Potassium molybdate (K 2 MoO 4 ) | Potassium tellurite (K 2 TeO 3 ) | Potassium tellurate (K 2 TeO 4 ) | Potassium tantalate (KTaO 3 ) | Potassium perxenate (K 4 XeO 6 ) | Potassium tungstate (K 2 WO 4 ) |
| Calcium | +2 | Calcium molybdate (CaMoO 4 ) | Calcium Tellurite (CaTeO 3 ) | Calcium tellurate (CaTeO 4 ) | Calcium tungstate (CaWO 4 ) | ||
| Rubidium | +1 | Rubidium molybdate (Rb 2 MoO 4 ) | Rubidium Tellurate (Rb 2 TeO 4 ) | Rubidium Tantalate (RbTaO 3 ) | Rubidium tungstate (Rb 2 WO 4 ) | ||
| strontium | +2 | Strontium molybdate (SrMoO 4 ) | Strontium Tellurite (SrTeO 3 ) | Strontium tellurate (SrTeO 4 ) | Strontium tungstate (SrWO 4 ) | ||
| Cesium | +1 | Cesium molybdate (Cs 2 MoO 4 ) | Cesium tellurate (Cs 2 TeO 4 ) | Cesium tantalate (CsTaO 3 ) | Cesium tungstate (Cs 2 WO 4 ) | ||
| barium | +2 | Barium molybdate (BaMoO 4 ) | Barium tellurite (BaTeO 3 ) | Barium tellurate (BaTeO 4 ) | Barium perxenate (Ba 2 XeO 6 ) | Barium tungstate (BaWO 4 ) |
List of inorganic bases
Hydroxides, hydrogen sulfides and amides
Hydroxides contain hydroxide ions as negatively charged ions . Hydrogen sulfides are salts of hydrogen sulfide . Ionic amides are derived from ammonia .
List of inorganic acids
Colloquial names (common names)
The following table lists common names with the corresponding systematic names ( IUPAC names ) of known inorganic salts alphabetically.