Aluminum diboride
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
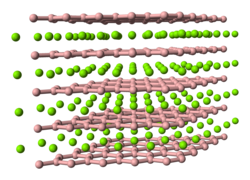
|
|||||||||||||||||||
| __ Al __ B | |||||||||||||||||||
| Space group |
P 6 / mmm (No. 191) |
||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Aluminum diboride | ||||||||||||||||||
| other names |
Aluminum boride (ambiguous) |
||||||||||||||||||
| Ratio formula | AlB 2 | ||||||||||||||||||
| Brief description |
black solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 48.60 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
3.172 g cm −3 |
||||||||||||||||||
| Melting point |
975 ° C (decomposition) |
||||||||||||||||||
| solubility |
reacts slowly with water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Aluminum diboride is an inorganic chemical compound of aluminum from the group of borides .
Extraction and presentation
Aluminum diboride can be obtained by reacting aluminum with boron in a vacuum at 800 ° C.
properties
Aluminum diboride is a dark solid that can also be in the form of shiny hexagonal plates. Its reaction behavior is very dependent on the particle size. At temperatures above 975 ° C it breaks down into aluminum and aluminum dodecaboride AlB 12 . It has a hexagonal crystal structure (a = 300.9 pm, c = 326.2 pm) with the space group P 6 / mmm (space group no. 191) and forms mixed crystals with the magnesium diboride and manganese diboride crystallizing in the same lattice .
See also
- Aluminum dodecaboride (AlB 12 )
Individual evidence
- ^ A b c d e Jean d'Ans, Ellen Lax, Roger Blachnik: Pocket book for chemists and physicists . Springer DE, 1998, ISBN 3-642-58842-5 , pp. 292 ( limited preview in Google Book search).
- ↑ a b Data sheet Aluminum diboride, −325 mesh from Sigma-Aldrich , accessed on January 27, 2014 ( PDF ).
- ↑ a b c Georg Brauer (ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 788.
- ^ HJ Becher: On the representation and stability of aluminum diboride. In: Journal of Inorganic and General Chemistry. 308, 1961, pp. 13-22, doi : 10.1002 / zaac.19613080104 .
