Bismuth (III) sulfide
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
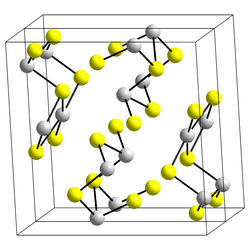
|
|||||||||||||||||||
| __ Bi 3+ __ S 2− | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Bismuth (III) sulfide | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Ratio formula | Bi 2 S 3 | ||||||||||||||||||
| Brief description |
dark brown or gray solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 514.16 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
|
||||||||||||||||||
| Melting point |
685 ° C (other source 850 ° C) |
||||||||||||||||||
| solubility |
almost insoluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−140.2 kJ mol −1 |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Bismuth (III) sulfide is a chemical compound made from bismuth and sulfur .
Occurrence
Bismuth sulfide occurs naturally in the mineral bismuthinite . It is believed that it also occurs as a thin layer on the mountains of Venus .
Extraction and presentation
Bismuth sulfide can be produced in a precipitation reaction from bismuth (III) salts with a hydrogen sulfide solution.
properties
Bismuth sulfide has an orthorhombic crystal structure with the space group Pnma (space group no. 62) and the lattice parameters a = 11.269 Å , b = 3.972 Å and c = 11.129 Å. In addition, with 1.6 · 10 −72 it has one of the smallest solubility products of all chemical compounds.
use
Bismuth sulfide as a mineral is used as a raw material for the production of bismuth. It is also used as an additive in brake linings , as a catalyst , as a component of flux in arc welding and as a component in the ignition charges of ammunition . Bismuth sulfide thin films and nanotubes are currently being researched as high-performance catalysts.
Individual evidence
- ↑ a b Bismuth (III) sulfide data sheet from AlfaAesar, accessed on January 29, 2010 ( PDF )(JavaScript required) .
- ↑ a b c d entry to bismuth (III) sulfide in the GESTIS database of IFA , retrieved on Feb. 14, 2017(JavaScript required) .
- ↑ a b webelements.com: Dibismuth Trisulphide
- ^ ESPI-Metals: Bismuth Sulphide
- ↑ M. Binnewies, E. Milke: Thermochemical Data of Elements and Compunds . 2nd Edition. Wiley-VCH, Weinheim 2002, ISBN 3-527-30524-6 , pp. 167 .
- ↑ Wissenschaft.de: Lead "snow" covers the mountains of Venus. February 11, 2004, accessed September 7, 2019 .
- ^ LF Lundegaard, E. Makovicky, T. Boffa Ballaran, T. Balic-Zunic: Crystal structure and cation ion electron pair activity of Bi 2 S 3 between 0 and 10 GPa. In: Physics and Chemistry of Minerals , 32, 2005, pp. 578-584, doi : 10.1007 / s00269-005-0033-2 .
- ^ The large table work - Cornelsen, page 139, ISBN 978-3-464-57143-9
- ↑ loradchemical.com: Bismuth Sulfide - Bi 2 S 3 , accessed June 14, 2013.
- ↑ S. MAHMOUD, AH EID, and H. OMAR: OPTICAL CHARACTERISTICS OF BISMUTH SULFIDE (Bi 2 S 3 ) THIN FILMS (PDF; 1.2 MB) In: FIZIKA A 6 (1997) 3, 111-120.

