Lithium chromate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
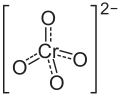
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Lithium chromate | ||||||||||||||||||
| Molecular formula | Li 2 CrO 4 | ||||||||||||||||||
| Brief description |
yellow odorless crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 129.87 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
2.15 g cm −3 (dihydrate) |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| solubility |
good in water (48.6 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Lithium chromate is the lithium salt of chromic acid . It is a strong oxidizing agent and like all chromates, it is toxic and carcinogenic.
presentation
Lithium chromate can be made from lithium carbonate and chromic acid.
properties
The dihydrate crystallizes in the orthorhombic crystal system in the space group P 2 1 2 1 2 1 (space group number 19) with the lattice constants a = 774.6 pm, b = 1201 pm and c = 550.9 pm as well as four formula units per unit cell .
The anhydrate crystallizes in the phenakite structure trigonal in the space group R 3 (space group no. 148) with the lattice parameters a = 14.01 Å and c = 9.41 Å and 18 formula units per unit cell .
If nitric acid is added to lithium chromate , lithium dichromate is formed .
When annealing with lithium oxide , lithium chromate (V) Li 3 CrO 4 is formed .
use
An aqueous solution of lithium chromate is used as a corrosion inhibitor and as an additive in batteries . The dihydrate is also used as an oxidizing agent.
Individual evidence
- ↑ a b c d e f Dale L. Perry, Sidney L. Phillips: Handbook of inorganic compounds . CRC Press, 1995, ISBN 978-0-8493-8671-8 , p. 223 ( limited preview in Google book search).
- ^ Entry on lithium chromate in the GESTIS material database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry Chromium (VI) compounds, with the exception of barium chromate and of compounds specified elsewhere in this Annex in the Classification and Labeling inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ R. Abegg, F. Auerbach, I. Koppel: Handbuch der inorganic Chemie . Verlag S. Hirzel, 1908, 2nd volume, 1st part, p. 140. Full text
- ↑ a b C. Rammelsberg: "About the isomorphism of the lithium salts with the potassium and sodium salts" in Pogg. Ann 1866 , 128 , p. 311 ff. Full text
- ^ Jean D'Ans, Ellen Lax: Pocket book for chemists and physicists. 3. Elements, inorganic compounds and materials, minerals, Volume 3. 4. Edition, Springer, 1997, ISBN 978-3-5406-0035-0 , p. 398 ( limited preview in the Google book search).
- ↑ H. Barlage, H. Jacobs: "Li 2 CrO 4 · 2H 2 O: Unusual hydrogen bonding and coordination of the O ligands of the anion CrO 4 2− " in Zeitschr. f. anorg. and allg. Chem. 1996 , 622 (4), pp. 721-723. doi : 10.1002 / zaac.19966220426
- ↑ ID Brown, R. Faggiani: "Refinement of the crystal structure of lithium chromate" in Acta Cryst. 1975 , B31 , pp. 2364-2365. doi : 10.1107 / S0567740875007625
- ^ KA Wilhelmi, O. Jonsson: X-Ray Studies on Some Alkali and Alkaline-Earth Chromates (V). In: Acta Chem. Scand. 1965, 19, doi : 10.3891 / acta.chem.scand.19-0177 , pp. 177-184.
- ↑ R. Scholder, H. Schwarz: "About Alkalichromate (V)" in the journal for inorganic and general chemistry 1963 , 326 (1-2) pp. 1-10. doi : 10.1002 / zaac.19633260102



