Ytterbium (III) chloride
| Crystal structure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| __ Yb 3+ __ Cl - | ||||||||||
| General | ||||||||||
| Surname | Ytterbium (III) chloride | |||||||||
| Ratio formula | YbCl 3 | |||||||||
| Brief description |
colorless solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | ||||||||||
| Physical state |
firmly |
|||||||||
| density |
2.57 g cm −3 |
|||||||||
| Melting point |
703 ° C |
|||||||||
| boiling point |
1900 ° C |
|||||||||
| solubility |
soluble in water |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Ytterbium (III) chloride is an inorganic chemical compound of ytterbium and is one of the chlorides . In addition to this, another ytterbium chloride is known with ytterbium (II) chloride .
Extraction and presentation
Ytterbium chloride is made by reacting ytterbium (III) oxide with either carbon tetrachloride or hot hydrochloric acid.
The anhydrous compound is produced by heating the water-containing compound in a stream of HCl at a slowly increasing temperature. At 350 ° C the substance is anhydrous.
properties
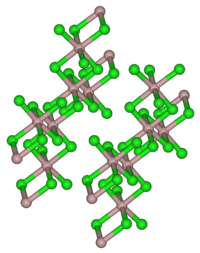
Ytterbium (III) chloride crystallizes in a cubic aluminum chloride layer structure. Smaller units such as [YbCl 6 ] 3− or Yb 2 Cl 6 are formed in the gas phase .
use
Catalyst in organic chemistry
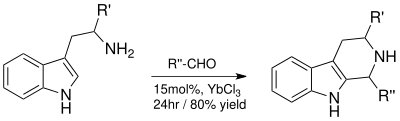
Ytterbium (III) chloride acts as a Lewis acid due to the single unpaired f-electron . This enables the compound to form complexes in transition states and thus to catalyze alkylation reactions such as aldol reactions and the Pictet-Spengler reaction .
- Aldol reaction
In the aldol reaction , ytterbium (III) chloride serves as an auxiliary catalyst in the palladium- catalyzed decarboxylating aldol reaction of an enolate with an aldehyde . The transition states A and B show the coordination of the ytterbium salt. For the decarboxylating aldol reaction described above with R = tert -butyl and R '= - (CH 2 ) 2 Ph, the comparison of the yields of different Lewis acids shows a particularly high yield for ytterbium (III) chloride.
| salt | % Yield of 2 |
|---|---|
| Iron (III) chloride | 40 |
| Zinc chloride | 68 |
| Copper (II) chloride | 40 |
| Lanthanum (III) chloride | 60 |
| Ytterbium (III) chloride | 93 |
- Pictet-Spengler reaction
As a Lewis acid, ytterbium (III) chloride catalyzes the Pictet-Spengler reaction to obtain tetrahydro- beta-carbolines , from which synthetic indole alkaloids are produced. It enables high yields and reduces the reaction time from days to 24 hours.
- Ester formation
The small size of the Yb 3+ ion enables rapid catalysis, but the selectivity is low. For example, the mono- acetylation of meso - 1,2-diols is fastest with ytterbium (III) chloride in two hours, whereas the chemoselectivity for the monoacetylated product is 50% low compared to cerium (III) chloride (23 h response time, 85%).
- Acetal formation
Ytterbium (III) chloride is a powerful catalyst for acetal formation with trimethyl orthoformate . Compared with cerium (III) chloride and erbium (III) chloride , the ytterbium salt is the most effective. It achieves high yields in a rapid reaction at room temperature with a wide variety of aldehydes.
NMR shift reagent
Ytterbium can be used as an NMR shift reagent , for example in membrane biology for tracking the movements of 39 K + and 23 Na + , which play an important role in nerve signals .
Individual evidence
- ↑ a b c data sheet ytterbium (III) chloride from AlfaAesar, accessed on February 28, 2012 ( PDF )(JavaScript required) .
- ↑ Data sheet Ytterbium (III) chloride hexahydrate, 99.9999% trace metals basis from Sigma-Aldrich , accessed on March 25, 2012 ( PDF ).
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-99.
- ^ A b John Harris, Walter Benenson, Horst Stöcker: Handbook of physics. Springer, 2002, ISBN 0-387-95269-1 , p. 781 ( limited preview in the Google book search).
- ↑ VF Goryushkin, SA Zalymova, AI Poshevneva. In: Russ. J. Inorg. Chem. 1990, 35, 12, pp. 1749-1752.
- ↑ Joerg Sebastian, Hans-Joachim Seifert: Ternary chlorides in the systems ACl / YbCl 3 (A = Cs, Rb, K). In: Thermochimica Acta. 318, 1998, pp. 29-37, doi : 10.1016 / S0040-6031 (98) 00326-8 .
- ↑ G. Jantsch, N. Skalla, H. Jawurek: “To the knowledge of the halides of the rare earths. V. - About the halides of ytterbium ”, in: Journal for inorganic and general chemistry , 1931 , 201 , pp. 207–220; doi : 10.1002 / zaac.19312010119 .
- ↑ Wei-Jyh Gau: Electrochemical and Spectroscopic Studies of ytterbium in the Aluminum Chloride-1-methyl-3-ethylimidazolium chlorides Room Temperature Molten Salt. In: Journal of The Electrochemical Society. 143, 1996, pp. 170-174, doi : 10.1149 / 1.1836403 .
- ↑ AD Chervonnyi, NA Chervonnaya: Thermodynamic Properties of ytterbium chloride. In: Russian Journal of Inorganic Chemistry. 2004, 49, 12, pp. 1889–1897 ( Abstract ( Memento of the original from March 4, 2016 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove it Note. ).
- ↑ a b c Sha Lou, John A. Westbrook, Scott E. Schaus: Decarboxylative Aldol Reactions of Allyl β-Keto Esters via Heterobimetallic Catalysis. In: Journal of the American Chemical Society. 126, 2004, pp. 11440-11441, doi : 10.1021 / ja045981k .
- ↑ a b Natarajan Srinivasan, A. Ganesan: Highly efficient Lewis acid-catalysed Pictet-Spengler reactions discovered by parallel screening. In: Chemical Communications. , Pp. 916-917, doi : 10.1039 / B212063A .
- ^ Paul A. Clarke: Selective mono-acylation of meso- and C 2 -symmetric 1,3- and 1,4-diols. In: Tetrahedron Letters. 43, 2002, pp. 4761-4763, doi : 10.1016 / S0040-4039 (02) 00935-8 .
- ^ Jean-Louis Luche, André Luis Gemal: Efficient synthesis of acetals catalysed by rare earth chlorides. In: Journal of the Chemical Society, Chemical Communications. 1978, p. 976, doi : 10.1039 / c39780000976 .
- ↑ Manajit K. Hayer, Frank G. Riddell: Shift reagents for 39 K Nmr. In: Inorganica Chimica Acta . 92, 1984, pp. L37-L39, doi : 10.1016 / S0020-1693 (00) 80044-4 .