Erbium (III) chloride
| Crystal structure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| __ Er 3+ __ Cl - | ||||||||||
| Space group |
C 2 / m (No. 12) |
|||||||||
| General | ||||||||||
| Surname | Erbium (III) chloride | |||||||||
| other names |
Erbium trichloride |
|||||||||
| Ratio formula | ErCl 3 | |||||||||
| Brief description |
pink solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 273.62 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
4.1 g cm −3 |
|||||||||
| Melting point |
|
|||||||||
| boiling point |
1500 ° C |
|||||||||
| solubility |
soluble in water |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Erbium (III) chloride is a chemical compound from the group of chlorides .
Extraction and presentation
Erbium (III) chloride can be obtained by reacting erbium (III) oxide or erbium (III) carbonate and ammonium chloride.
The hexahydrate is created by the reaction of erbium with hydrochloric acid . This can be converted to the anhydrate form by reaction with thionyl chloride .
Erbium (III) chloride can also be synthesized directly from the elements erbium and chlorine .
properties
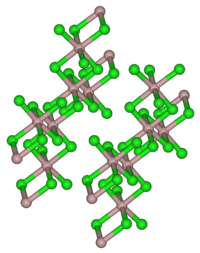
Erbium (III) chloride is an almost colorless, slightly pinkish-red solid. Its hexahydrate is a pink, hygroscopic solid. Both are soluble in water. Erbium (III) chloride has a monoclinic crystal structure with the space group C 2 / m (space group no. 12) corresponding to that of aluminum (III) chloride . The hexahydrate also has a monoclinic crystal structure with the space group P 2 / n (no. 13, position 2) . Erbium (III) chloride is a good catalyst for the acylation of alcohols and phenols.
use
Erbium (III) chloride is used to make pure erbium. It can also be used as a starting material for new edge-bridged octahedral M6 cluster compounds such as CsErTa 6 Cl 18 , for which electron and stability studies are carried out.
Individual evidence
- ↑ a b c d Jean D'Ans, Ellen Lax: Pocket book for chemists and physicists . 2007, ISBN 978-3-540-60035-0 , pp. 444 ( limited preview in Google Book search).
- ↑ a b c d e f g h data sheet Erbium (III) chloride, anhydrous, powder, 99.9% trace metals basis from Sigma-Aldrich , accessed on April 30, 2012 ( PDF ).
- ↑ Datasheet Erbium (III) chloride hexahydrate, 99.995% trace metals basis from Sigma-Aldrich , accessed on April 30, 2012 ( PDF ).
- ↑ a b Georg Brauer (Ed.), With the collaboration of Marianne Baudler u a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 897.
- ↑ Web elements: Erbium
- ↑ Renato Dalpozzo, Antonio De Nino, Loredana Maiuolo, Manuela Oliverio, Antonio Procopio, Beatrice Russo, Amedeo Tocci: Erbium (III) Chloride: a Very Active Acylation Catalyst , Australian Journal of Chemistry, 2006, 60 (1), p. 75 -79, doi : 10.1071 / CH06346 .
- ^ John Emsley: Nature's building blocks: an AZ guide to the elements . Oxford University Press, 2003, ISBN 978-0-19-850340-8 , pp. 137 ( limited preview in Google Book search).




