Ytterbium (III) oxide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
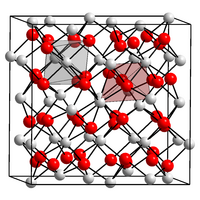
|
||||||||||||||||
| __ Yb 3+ __ O 2− | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Ytterbium (III) oxide | |||||||||||||||
| other names |
|
|||||||||||||||
| Ratio formula | Yb 2 O 3 | |||||||||||||||
| Brief description |
White dust |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 394.08 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
9.17 g cm −3 |
|||||||||||||||
| Melting point |
2355 ° C |
|||||||||||||||
| boiling point |
4070 ° C |
|||||||||||||||
| solubility |
soluble in acids |
|||||||||||||||
| Refractive index |
1.93542 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Ytterbium (III) oxide is a chemical compound from the group of oxides .
Occurrence
Ytterbium (III) oxide occurs naturally in traces in the mineral gadolinite . It was isolated from this in 1878 by Jean Charles Galissard de Marignac .
Extraction and presentation
Ytterbium (III) oxide can be obtained by reacting ytterbium with oxygen .
It can also be obtained by thermal decomposition of ytterbium carbonate or ytterbium oxalate at temperatures around 700 ° C.
properties
Ytterbium (III) oxide is a white powder. Like the other trivalent oxides of the heavier lanthanides, it crystallizes in a lanthanide-C cubic structure. It reacts with carbon tetrachloride or hot hydrochloric acid to form ytterbium (III) chloride .
use
Ytterbium (III) oxide is used as an additive for special alloys and dielectric ceramic materials, as a catalyst and in special glasses. It can also be used as an additive for carbon electrodes in arc lamps , which produce a very bright light.
Individual evidence
- ↑ a b c d e f data sheet Ytterbium (III) oxide, 99.99% trace metals basis from Sigma-Aldrich , accessed on January 28, 2019 ( PDF ).
- ^ A b Thomas Bauer: Thermophotovoltaics: Basic Principles and Critical Aspects of System Design . Springer Berlin Heidelberg, 2011, ISBN 978-3-642-19964-6 , p. 20 ( limited preview in Google Book search).
- ^ A b George WA Milne: Gardner's commercially important chemicals . John Wiley & Sons, 2005, ISBN 978-0-471-73518-2 , pp. 767 ( limited preview in Google Book search).
- ↑ O. Medenbach et al., Refractive index and optical dispersion of rare earth oxides using a small-prism technique, J. Opt. A: Pure Appl. Opt., 2001, 3, pp. 174-177; doi : 10.1088 / 1464-4258 / 3/3/303 .
- ^ A b Robert E. Krebs: The history and use of our earth's chemical elements: a reference guide . Greenwood Pub Group, 2006, ISBN 978-0-313-33438-2 , pp. 302 ( limited preview in Google Book search).
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1938-1944.
- ↑ Gerd Meyer, Lester R. Morss: Synthesis of lanthanide and actinide compounds . Springer Netherlands, 1990, ISBN 978-0-7923-1018-1 , pp. 196 ( limited preview in Google Book search).
- ↑ VF Goryushkin, SA Zalymova, AI Poshevneva. In: Russ. J. Inorg. Chem. 1990, 35, 12, pp. 1749-1752.
- ↑ Joerg Sebastian, Hans-Joachim Seifert: Ternary chlorides in the systems ACl / YbCl3 (A = Cs, Rb, K). In: Thermochimica Acta. 318, 1998, pp. 29-37, doi : 10.1016 / S0040-6031 (98) 00326-8 .




