Arsenic (V) fluoride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
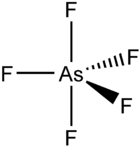
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Arsenic (V) fluoride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | AsF 5 | |||||||||||||||
| Brief description |
colorless gas that smokes in air |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 169.91 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
2.14 g cm −3 (liquid at boiling point) |
|||||||||||||||
| Melting point |
−79.8 ° C |
|||||||||||||||
| boiling point |
−52.8 ° C |
|||||||||||||||
| solubility |
soluble in ethanol , diethyl ether and benzene |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Arsenic pentafluoride , AsF 5 , is a chemical compound consisting of the elements arsenic and fluorine , in which arsenic has the + V oxidation state . Like antimony (V) fluoride, it is a strong Lewis acid , a fluorinating and oxidizing agent .
presentation
Arsenic pentafluoride can be prepared directly from the elements by reaction.
Further methods are the fluorination of arsenic (III) fluoride or arsenic oxides with elemental fluorine .
It can also be obtained without the use of elemental fluorine from arsenic trifluoride and antimony pentafluoride with bromine as a catalyst .
properties
Physical Properties
Arsenic pentafluoride is a colorless gas at room temperature . It has a trigonal-bipyramidal geometry.
Chemical properties
Arsenic pentafluoride is hydrolyzed by water. It goes through reaction with fluoride - donors as with sulfur tetrafluoride in the octahedral via built hexafluoroarsenate (V):
- .
Different ionic compounds are formed with xenon difluoride depending on the mixing ratio.
literature
- Otto Ruff, A. Braida, O. Bretschneider, W. Menzel, H. Plaut: The representation, vapor pressures and densities of the BF 3 , AsF 5 and BrF 3 . In: Journal of Inorganic and General Chemistry. 206, 1932, pp. 59-64, doi : 10.1002 / zaac.19322060108 .
Individual evidence
- ↑ Entry on arsenic fluoride. In: Römpp Online . Georg Thieme Verlag, accessed on July 14, 2014.
- ↑ a b c d Entry on arsenic (V) fluoride in the GESTIS substance database of the IFA , accessed on February 16, 2017(JavaScript required) .
- ↑ a b G. Brauer (Ed.), Handbook of Preparative Inorganic Chemistry 2nd ed., Vol. 1, Academic Press 1963, pp. 198-9.
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry arsenic compounds, with the exception of those named in this appendix in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA) on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Ralf Steudel : Chemistry of Nonmetals, Syntheses - Structures - Bonding - Use , 4th Edition, 2014 Walter de Gruyter GmbH & Co. KG, Berlin / Boston, ISBN 978-3-11-030439-8 , p. 570, ( accessed via De Gruyter Online).




![{\ displaystyle \ mathrm {AsF_ {5} + SF_ {4} \ longrightarrow SF_ {3} ^ {+} [AsF_ {6}] ^ {-}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9ee1d1d083216e6e15ff78f052c1dc1358037ee2)
![{\ displaystyle \ mathrm {2 \, XeF_ {2} + AsF_ {5} \ rightarrow [Xe_ {2} F_ {3}] [AsF_ {6}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7908536e1314d65cc30c1cbcee6f034181c805e7)
![{\ displaystyle \ mathrm {XeF_ {2} + AsF_ {5} \ rightarrow [XeF] [AsF_ {6}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a90aabf3a158d87480222a600ccd1d47c1f21d15)
![{\ displaystyle \ mathrm {XeF_ {2} +2 \, AsF_ {5} \ rightarrow [XeF] [As_ {2} F_ {11}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4059be4656983f8a65dc2d53e40cdd0db85c91bc)