Arsenic pentachloride
| Crystal structure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Arsenic pentachloride | |||||||||
| other names |
Arsenic (V) chloride |
|||||||||
| Ratio formula | AsCl 5 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 252.19 g mol −1 | |||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Arsenic pentachloride is a metastable inorganic chemical compound of arsenic from the group of chlorides .
Extraction and presentation
Arsenic pentachloride can be obtained by photochemical chlorination of arsenic (III) chloride at −105 ° C.
properties
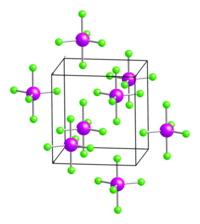
Arsenic pentachloride is a metastable compound; its decomposition is catalyzed by hydrogen chloride . The noticeable instability of the connection is z. Partly attributed to the transition metal contraction, partly to the weakness of the AsCl bond. Above −50 ° C it breaks down again into arsenic (III) chloride and chlorine. The compound has a trigonal bipyramidal geometry and crystallizes in the orthorhombic space group Pmmn (space group no. 59) .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ A b Arnold F. Holleman, Egon Wiberg: Textbook of inorganic chemistry . Walter de Gruyter, 1995, ISBN 978-3-11-012641-9 , pp. 801 ( limited preview in Google Book search).
- ↑ Konrad Seppelt : Arsenic pentachloride, AsCl5. In: Journal of Inorganic and General Chemistry . 434, 1977, p. 5, doi : 10.1002 / zaac.19774340101 .
- ^ Paul Gouda: Arsenic, Selenium, Antimony ultra-trace analysis . iUniverse, 2012, ISBN 1-4759-4803-4 , pp. 47 ( limited preview in Google Book search).
- ↑ Silvia Haupt, Konrad Seppelt: Solid State Structures of AsCl5 and SbCl5 . In: Journal of Inorganic and General Chemistry . tape 628 , no. 4 , May 2002, p. 729-734 , doi : 10.1002 / 1521-3749 (200205) 628: 4 <729 :: AID-ZAAC729> 3.0.CO; 2-E .
