Calcium bromate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 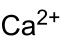
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Calcium bromate | |||||||||||||||
| other names |
E924b |
|||||||||||||||
| Molecular formula | Ca (BrO 3 ) 2 | |||||||||||||||
| Brief description |
white crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 295.88 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
180 ° C |
|||||||||||||||
| solubility |
soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Calcium bromate is the calcium salt of bromic acid .
presentation
Calcium bromate can be made by dissolving calcium carbonate in bromic acid.
Calcium bromate is also formed by the salt formation reaction between calcium hydroxide and bromic acid.
properties
Calcium bromate crystallizes as a monohydrate in monoclinic columns that are isomorphic with strontium bromate and barium bromate . They give off their crystal water between 130 and 150 ° C. At 270-300 ° C, calcium bromate decomposed to calcium .
Individual evidence
- ↑ a b c d e data sheet calcium bromate at AlfaAesar, accessed on April 30, 2017 ( PDF )(JavaScript required) .
- ↑ a b R. Abegg, F. Auerbach: "Handbuch der inorganic Chemie". Verlag S. Hirzel, Vol. 2, 1908, p. 129; Full text
- ^ A b V. A. Stenger, RM van Effen, LC Walker: "Solubility of Calcium Bromate in Water" in J. Chem. Eng. Data 2002 , 47 (3), pp. 618-619. doi : 10.1021 / je010264g




