Molybdenum (IV) chloride
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
|
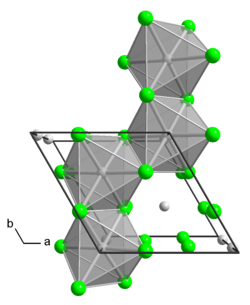
__ Mo 4+ __ Cl - crystal structure of α-MoCl 4 |
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Molybdenum (IV) chloride | ||||||||||||||||||
| other names |
Molybdenum tetrachloride |
||||||||||||||||||
| Ratio formula | MoCl 4 | ||||||||||||||||||
| Brief description |
black solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 237.75 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
3.193 g cm −3 |
||||||||||||||||||
| Melting point |
272 ° C (decomposition) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Molybdenum (IV) chloride is an inorganic chemical compound of molybdenum from the group of chlorides .
Extraction and presentation
Molybdenum (IV) chloride can be obtained by reacting molybdenum (V) chloride with benzene , molybdenum (III) chloride or tetrachlorethylene .
properties
Molybdenum (IV) chloride is a paramagnetic , light-, air- and moisture-sensitive black powder or black-brown, hexagonal columns. It is extremely susceptible to hydrolysis and dissolves in water, ethanol and ether without leaving any residue with a yellow to red-brown color . When heated to over 180 ° C in a vacuum, it is volatile with partial decomposition into molybdenum (III) chloride and molybdenum (V) chloride. It has a trigonal crystal structure with the space group P 3 1 c (space group no. 163) , a = 605.8 pm, c = 1167.4 pm. The compound (α-form with chains of trans-edge-linked MoCl 6 octahedra) is converted at 250 ° C into the β-form, which consists of rings of six cis-edge-linked MoCl 6 octahedra. When dissolved in concentrated hydrochloric acid, it forms complex salts with alkali chlorides, such as the apple-green cesium pentachlorooxomolybdate (V) Cs 2 [MoOCl 5 ].
Individual evidence
- ↑ a b c d e Georg Brauer: Handbook of preparative inorganic chemistry . 3., reworked. Edition. tape III . Enke, Stuttgart 1981, ISBN 3-432-87823-0 , pp. 1533 .
- ↑ a b c d Jean d'Ans, Ellen Lax, Roger Blachnik: Pocket book for chemists and physicists . Springer DE, 1998, ISBN 3-642-58842-5 , pp. 574 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ EL McCann III and TM Brown: Molybdenum (IV) chloride . In: Robert W. Parry (Ed.): Inorganic Syntheses . tape 12 . McGraw-Hill Book Company, Inc., 1970, ISBN 07-048517-8 ( defective ) , p. 181-186 (English).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 1470.


