Dicobalt carbide
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
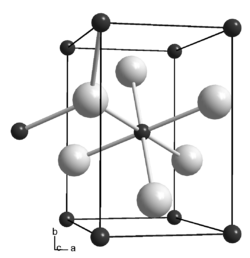
|
|||||||
|
__ Co __ C Metal-to-metal bonds are not shown |
|||||||
| General | |||||||
| Surname | Dicobalt carbide | ||||||
| other names |
Cobalt carbide (ambiguous) |
||||||
| Ratio formula | Co 2 C | ||||||
| Brief description |
gray solid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 129.88 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| density |
7.76 g cm −3 |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Dicobalt carbide is an inorganic chemical compound of cobalt from the group of carbides .
Extraction and presentation
Dicobalt carbide can be obtained by reacting cobalt with carbon monoxide at 220 ° C.
properties
Dicobalt carbide is a metallic, gray solid. It decomposes between 260 and 310 ° C and is converted into a hexagonal shape by hydrogen between 198 and 275 ° C, by nitrogen between 297 and 369 ° C, and by carbon dioxide between 364 and 540 ° C. It has an orthorhombic crystal structure with the space group Pmnn (space group no. 58, position 3) and the lattice parameters a = 288 pm, b = 445 pm, c = 436 pm and two formula units per unit cell . In the crystal structure, each carbon atom is octahedral surrounded by six cobalt atoms, each cobalt atom trigonal-planar by three carbon atoms. In addition to dicobalt carbide, there is also tricobalt carbide Co 3 C (CAS number: 12011-59-5). It is only stable in the temperature range between 500 and 800 ° C, has a density of 8.4 g · cm −3 and a crystal structure isotypic with triiron carbide.
use
Dicobalt carbide is formed when cobalt catalysts are used in the Fischer-Tropsch synthesis and influences this reaction.
literature
- Yong-Hui Zhao, Hai-Yan Su, Keju Sun, Jinxun Liu, Wei-Xue Li: Structural and electronic properties of cobalt carbide Co 2 C and its surface stability: Density functional theory study. In: Surface Science. 606, 2012, pp. 598-604, doi : 10.1016 / j.susc.2011.11.025 .
Individual evidence
- ↑ a b c d Georg Brauer: Handbook of preparative inorganic chemistry . 3., reworked. Edition. tape III . Enke, Stuttgart 1981, ISBN 3-432-87823-0 , pp. 1674 .
- ↑ a b c d Jean d'Ans, Ellen Lax, Roger Blachnik: Pocket book for chemists and physicists . Springer DE, 1998, ISBN 3-642-58842-5 , pp. 380 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ BH Davis, Mario L. Occelli: Advances in Fischer-Tropsch Synthesis, Catalysts, and Catalysis . CRC Press, 2010, ISBN 1-4200-6257-3 , pp. 67 ( limited preview in Google Book search).
- ↑ Jianmin Xiong, Yunjie Ding, Tao Wang, L. i. Yan, Weimiao Chen, Hejun Zhu, Yuan Lu: The formation of Co 2 C species in activated carbon supported cobalt-based catalysts and its impact on Fischer-Tropsch reaction. In: Catalysis Letters. 102, 2005, pp. 265-269, doi : 10.1007 / s10562-005-5867-1 .
